Stay Ahead in Fast-Growing Economies.
Browse Reports NowPreeclampsia Diagnostics Market- Size & Upcoming Industry Trends 2024-2032
The preeclampsia diagnostics market is highly developed and is constantly expanding, mainly due to the enhanced focus on the condition of pregnant women and early signs of pregnancy complications. Preeclampsia develops when pregnancy lasting over 20weeks and is associated with high blood pressures,organ damage and complications to both the mother and the unborn child.
IMR Group
Description
Preeclampsia Diagnostics Market Synopsis:
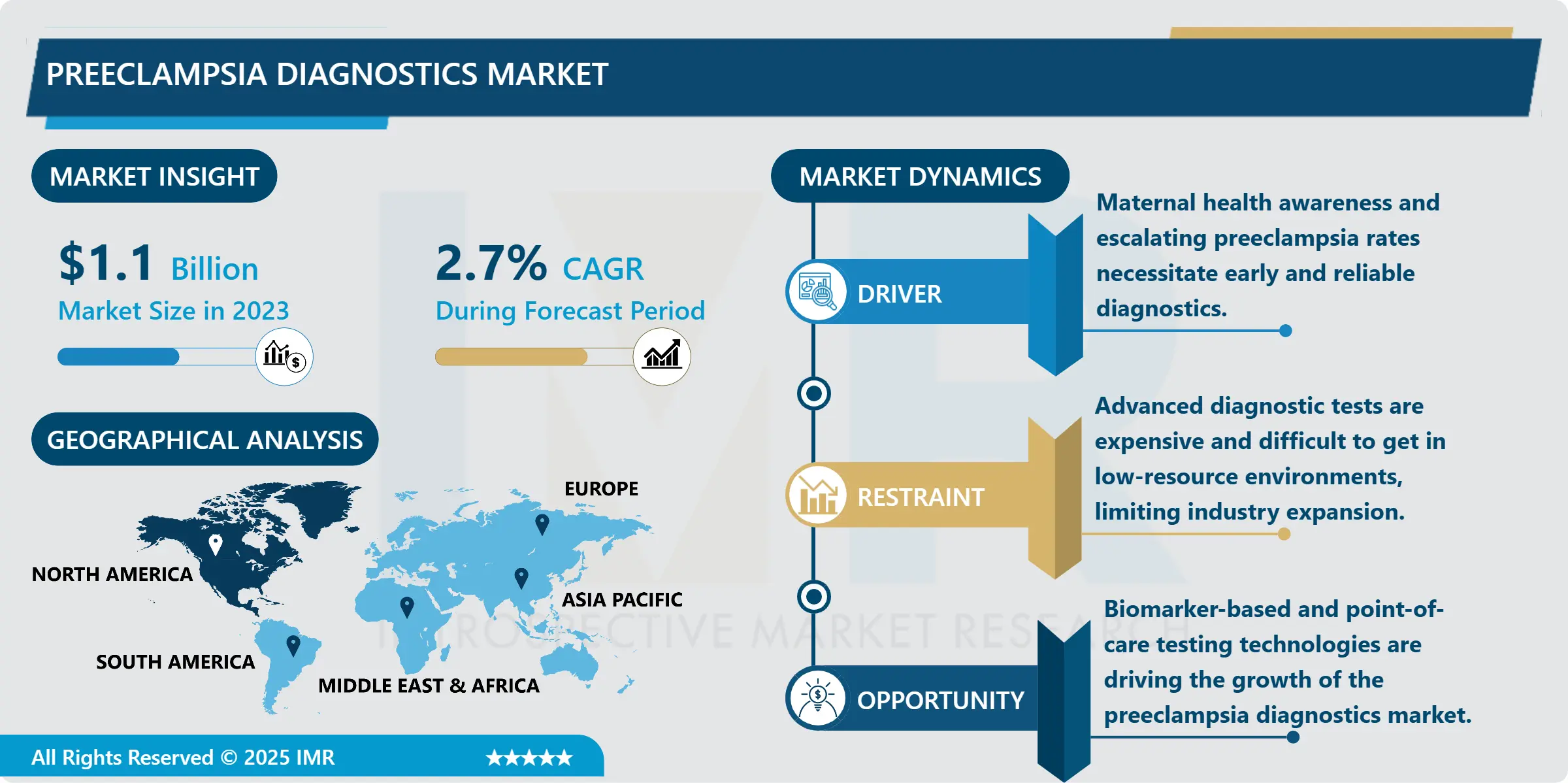
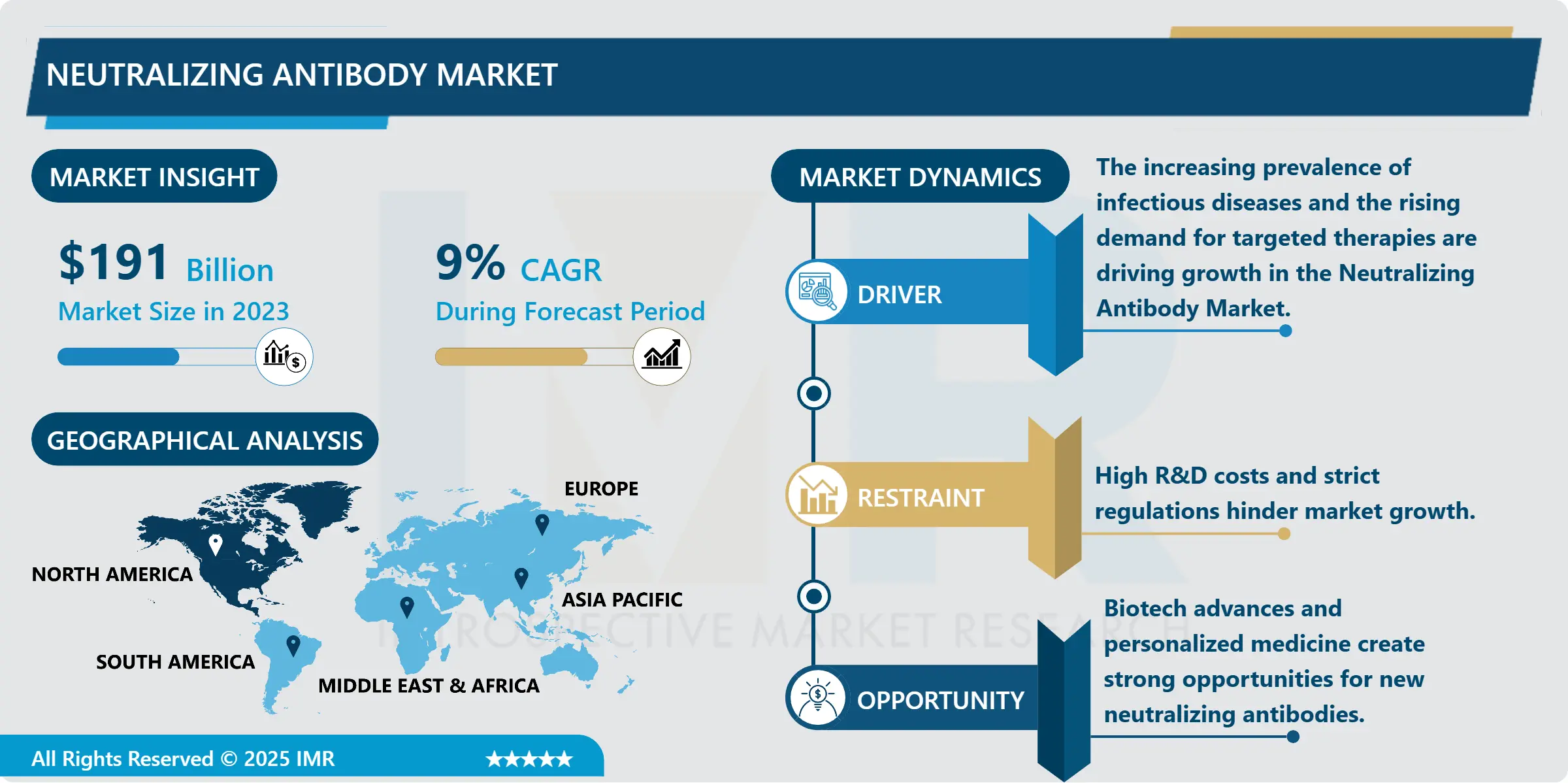
Preeclampsia Diagnostics Market Size Was Valued at USD 1.1 Billion in 2023, and is Projected to Reach USD 1.4 Billion by 2032, Growing at a CAGR of 2.7% From 2024-2032.
The preeclampsia diagnostics market is highly developed and is constantly expanding, mainly due to the enhanced focus on the condition of pregnant women and early signs of pregnancy complications. Preeclampsia develops when pregnancy lasting over 20weeks and is associated with high blood pressures,organ damage and complications to both the mother and the unborn child. Preeclampsia should therefore be diagnosed as early as possible since treatment in its early stages can go a long way in preventing future health complications and the general well being of the mother and child. Market demand is being promoted by new methods of diagnosis, biomarker-based blood tests, and higher-quality imaging that enables doctors to diagnose preeclampsia at an earlier stage of pregnancy.
Consistent with this paradigm shift, there are now available new diagnostics probes for biomarker assays and genetic tests, which have revolutionized the screening for preeclampsia.. Pregnant woman really prefer non-invasive diagnostic techniques and many organizations and research facility are working for the same. Furthermore, point of care testing is emerging, the results allow for quicker diagnosis and instant clinical decisions. Increasing developments in technology and further research into biomarkers unique to the disease such as preeclampsia is expected to foster the growth of this market particularly in the developed countries.• Nevertheless, there is the problem of inadequate diagnostic tools in LMIC and expensive state-of-art diagnostic tests.and more accessible. Many companies and research institutions are focusing on developing non-invasive diagnostic methods, as they offer a safer and more convenient option for pregnant women. In addition, point-of-care testing is gaining traction, enabling faster diagnosis and immediate clinical decisions. Technological advancements and growing research into biomarkers specific to preeclampsia are expected to support the growth of this market, especially in regions with strong healthcare infrastructure.
However, challenges persist, including limited access to diagnostic resources in low- and middle-income countries and the high costs associated with advanced diagnostic tests. However, the growth of preeclampsia diagnostics market is restricted by some of these factors, the increasing healthcare costs and campaigns that enhance the awareness of maternal health give this market the potential for growth. Global and regional governments and organizations are also focusing on extending maternal care and health, and thus newly investing in preeclampsia research and diagnostic tools. With increased understanding of preeclampsia and the effects it has on both maternal and fetal health, diagnostics market has potential for further development with improved and available testing methods for maternal health care.
Preeclampsia Diagnostics Market Trend Analysis:
Advancements in Biomarker-Based Diagnostics
Mergers and acquisitions also are being witnessed, and one of the major characteristics of this market area is biomarker based approach to preeclampsia diagnostics.. Scientists are discovering which biomarkers can be a sign of preeclampsia early enough so that it can be dealt with appropriately. The biomarkers include soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF) which is being incorporated in diagnostic platforms that can solve all the problems facing traditional methods. This strong growth can be attributed to the rising number of studies and clinical trials assessing biomarkers and fostering development of new, better diagnostic tools with enhanced early detection rates.
Rising Adoption of Point-of-Care Testing
Another upcoming area of concern in the preeclampsia diagnostics market is the current usage of point-of-care (POC) testing solutions.. Pocket sized POC devices can be used prominently within hospitals, clinics and even at home to facilitate real time testing. These are portable, easy to use devices that fill the gap in preeclampsia diagnostics especially in situations where laboratory based diagnostics are a novelty. The benefits accruing from POC testing include enhanced convenience and speed of the test result—and therefore improved speed of the clinical decision-making process, which in the case of preeclampsia is critical to the management of the pregnancy to safeguard maternal and fetal health. Over time, the steady market assimilation of automated technology is deemed effective to increase the usefulness of the POC solutions in diagnostic strategy.
Preeclampsia Diagnostics Market Segment Analysis:
Preeclampsia Diagnostics Market is Segmented on the basis of Test type, Product, End User, and Region.
By Test type, Blood Tests segment is expected to dominate the market during the forecast period
As far as test type is concerned blood tests and urine analysis have vast importance in diagnosing preeclampsia in the diagnostics market.. There is also use blood biochemistry tests of peptides such as soluble fms-like tyrosine kinase 1 (sFlt-1), placental growth factor (PlGF) and other proteins that suggest preeclampsia. These tests give the physician an indication of the patient cardiovascular and renal status and assist in early diagnosis of the condition. On the other hand, urine examination is frequently used to screen for high protein, a constituent that is characteristic of preeclampsia. These diagnostic methods are imperative to identify the factors that put a woman at risk for developing preeclampsia, and managing the risks also play a tremendous role in improving the outcomes of the mother and the baby.
By End User, Specialty Clinics segment expected to held the largest share
Preeclampsia diagnostics market is classified on the basis of end users such as hospitals, specialty clinics, diagnostics centers and others.. Hospitals have complex facilities which makes it easy for professional midwifes, skilled health personnel and will be able to handle high risk pregnancies. Such market players as specialty clinics, concerned with maternal and prenatal care, are also major ones due to the focus on individual diagnostics and treatment of diseases, including preeclampsi Diagnostic centers are very useful in the diagnosis of maternal conditions since these specific centers offer mere tests and screening. Furthermore, other end users such as home healthcare and outpatient care is also emerged universally as a result of the rising the demand for home-based testing and affordable prenatal care. Some of the factors that affect the market across these segments include; access to health care, uptake of enhanced diagnostics, rising focus on preventative and maternal care.
Preeclampsia Diagnostics Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
North America is expected to have the largest market share for preeclampsia diagnostics over the above forecast period due to the advancements in healthcare infrastructure, high focus of mothers for their health, and increased emphasis on research in this particular field.. Due to the well-developed healthcare systems, increased embracement of innovative techniques by healthcare facilities especially in the United States and Canada, biomarker diagnostic technologies for multiplex panels and point-of-care solutions are widely adoptable. Furthermore, the growth of the government and health care policies aimed at increasing the maternal and fetal health put additional pressure on demand for diagnostics of preeclampsia. With a particular emphasis on risk assessment and prevention of maternal mortality, North America is set to become the leader in controlling the market within the next couple of years.
Active Key Players in the Preeclampsia Diagnostics Market:
F. Hoffmann-La Roche Ltd (Switzerland)
PerkinElmer Inc. (Massachusetts, US)
DRG INSTRUMENTS GMBH (Germany)
Thermo Fisher Scientific Inc. (US)
Diabetomics, Inc. (US)
Metabolomic Diagnostics Ltd. (Ireland)
Sera Prognostics (US)
Siemens Healthineers AG (Germany)
Bayer AG (Germany)
Abbott Laboratories (US)
Agilent Technologies, Inc. (US)
Bio-Rad Laboratories, Inc. (US)
Danaher Corporation (US)
Illumina, Inc. (US)
Natera, Inc. (US)
GE Healthcare (US)
Medtronic plc (Ireland)
Fujifilm Holdings Corporation (Japan)
Mindray Bio-Medical Electronics Co., Ltd. (China)
AstraZeneca plc (UK)
Other Active Players
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Industry Dynamics and Opportunity Analysis
3.1.1 Growth Drivers
3.1.2 Limiting Factors
3.1.3 Growth Opportunities
3.1.4 Challenges and Risks
3.2 Market Trend Analysis
3.3 Strategic Pestle Overview
3.4 Porter’s Five Forces Analysis
3.5 Industry Value Chain Mapping
3.6 Regulatory Framework
3.7 Princing Trend Analysis
3.8 Patent Analysis
3.9 Technology Evolution
3.10 Investment Pockets
3.11 Import-Export Analysis
Chapter 4: Preeclampsia Diagnostics Market by Test type
4.1 Preeclampsia Diagnostics Market Snapshot and Growth Engine
4.2 Preeclampsia Diagnostics Market Overview
4.3 Blood Tests
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Blood Tests: Geographic Segmentation Analysis
4.4 and Urine Analysis
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 and Urine Analysis: Geographic Segmentation Analysis
Chapter 5: Preeclampsia Diagnostics Market by Product
5.1 Preeclampsia Diagnostics Market Snapshot and Growth Engine
5.2 Preeclampsia Diagnostics Market Overview
5.3 Instruments
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Instruments: Geographic Segmentation Analysis
5.4 and Consumables
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.4.3 and Consumables: Geographic Segmentation Analysis
Chapter 6: Preeclampsia Diagnostics Market by End User
6.1 Preeclampsia Diagnostics Market Snapshot and Growth Engine
6.2 Preeclampsia Diagnostics Market Overview
6.3 Hospitals
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.3.3 Hospitals: Geographic Segmentation Analysis
6.4 Specialty Clinics
6.4.1 Introduction and Market Overview
6.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.4.3 Specialty Clinics: Geographic Segmentation Analysis
6.5 Diagnostic Centers & Others
6.5.1 Introduction and Market Overview
6.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.5.3 Diagnostic Centers & Others: Geographic Segmentation Analysis
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Preeclampsia Diagnostics Market Share by Manufacturer (2023)
7.1.3 Concentration Ratio(CR5)
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 F. HOFFMANN-LA ROCHE LTD (SWITZERLAND)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Operating Business Segments
7.2.5 Product Portfolio
7.2.6 Business Performance
7.2.7 Key Strategic Moves and Recent Developments
7.3 PERKINELMER INC. (MASSACHUSETTS
7.4 US)
7.5 DRG INSTRUMENTS GMBH (GERMANY)
7.6 THERMO FISHER SCIENTIFIC INC. (US)
7.7 DIABETOMICS INC. (US)
7.8 METABOLOMIC DIAGNOSTICS LTD. (IRELAND)
7.9 SERA PROGNOSTICS (US)
7.10 SIEMENS HEALTHINEERS AG (GERMANY)
7.11 BAYER AG (GERMANY)
7.12 ABBOTT LABORATORIES (US)
7.13 AGILENT TECHNOLOGIES INC. (US)
7.14 BIO-RAD LABORATORIES INC. (US)
7.15 DANAHER CORPORATION (US)
7.16 ILLUMINA INC. (US)
7.17 NATERA INC. (US)
7.18 GE HEALTHCARE (US)
7.19 MEDTRONIC PLC (IRELAND)
7.20 FUJIFILM HOLDINGS CORPORATION (JAPAN)
7.21 MINDRAY BIO-MEDICAL ELECTRONICS CO. LTD. (CHINA)
7.22 ASTRAZENECA PLC (UK)
7.23 OTHER ACTIVE PLAYERS KEY PLAYERS
7.24 OTHER ACTIVE PLAYERS
Chapter 8: Global Preeclampsia Diagnostics Market By Region
8.1 Overview
8.2. North America Preeclampsia Diagnostics Market
8.2.1 Historic and Forecasted Market Size by Segments
8.2.2 Historic and Forecasted Market Size By Test type
8.2.2.1 Blood Tests
8.2.2.2 and Urine Analysis
8.2.3 Historic and Forecasted Market Size By Product
8.2.3.1 Instruments
8.2.3.2 and Consumables
8.2.4 Historic and Forecasted Market Size By End User
8.2.4.1 Hospitals
8.2.4.2 Specialty Clinics
8.2.4.3 Diagnostic Centers & Others
8.2.5 Historic and Forecast Market Size by Country
8.2.5.1 US
8.2.5.2 Canada
8.2.5.3 Mexico
8.3. Eastern Europe Preeclampsia Diagnostics Market
8.3.1 Historic and Forecasted Market Size by Segments
8.3.2 Historic and Forecasted Market Size By Test type
8.3.2.1 Blood Tests
8.3.2.2 and Urine Analysis
8.3.3 Historic and Forecasted Market Size By Product
8.3.3.1 Instruments
8.3.3.2 and Consumables
8.3.4 Historic and Forecasted Market Size By End User
8.3.4.1 Hospitals
8.3.4.2 Specialty Clinics
8.3.4.3 Diagnostic Centers & Others
8.3.5 Historic and Forecast Market Size by Country
8.3.5.1 Bulgaria
8.3.5.2 The Czech Republic
8.3.5.3 Hungary
8.3.5.4 Poland
8.3.5.5 Romania
8.3.5.6 Rest of Eastern Europe
8.4. Western Europe Preeclampsia Diagnostics Market
8.4.1 Historic and Forecasted Market Size by Segments
8.4.2 Historic and Forecasted Market Size By Test type
8.4.2.1 Blood Tests
8.4.2.2 and Urine Analysis
8.4.3 Historic and Forecasted Market Size By Product
8.4.3.1 Instruments
8.4.3.2 and Consumables
8.4.4 Historic and Forecasted Market Size By End User
8.4.4.1 Hospitals
8.4.4.2 Specialty Clinics
8.4.4.3 Diagnostic Centers & Others
8.4.5 Historic and Forecast Market Size by Country
8.4.5.1 Germany
8.4.5.2 UK
8.4.5.3 France
8.4.5.4 Netherlands
8.4.5.5 Italy
8.4.5.6 Russia
8.4.5.7 Spain
8.4.5.8 Rest of Western Europe
8.5. Asia Pacific Preeclampsia Diagnostics Market
8.5.1 Historic and Forecasted Market Size by Segments
8.5.2 Historic and Forecasted Market Size By Test type
8.5.2.1 Blood Tests
8.5.2.2 and Urine Analysis
8.5.3 Historic and Forecasted Market Size By Product
8.5.3.1 Instruments
8.5.3.2 and Consumables
8.5.4 Historic and Forecasted Market Size By End User
8.5.4.1 Hospitals
8.5.4.2 Specialty Clinics
8.5.4.3 Diagnostic Centers & Others
8.5.5 Historic and Forecast Market Size by Country
8.5.5.1 China
8.5.5.2 India
8.5.5.3 Japan
8.5.5.4 South Korea
8.5.5.5 Malaysia
8.5.5.6 Thailand
8.5.5.7 Vietnam
8.5.5.8 The Philippines
8.5.5.9 Australia
8.5.5.10 New Zealand
8.5.5.11 Rest of APAC
8.6. Middle East & Africa Preeclampsia Diagnostics Market
8.6.1 Historic and Forecasted Market Size by Segments
8.6.2 Historic and Forecasted Market Size By Test type
8.6.2.1 Blood Tests
8.6.2.2 and Urine Analysis
8.6.3 Historic and Forecasted Market Size By Product
8.6.3.1 Instruments
8.6.3.2 and Consumables
8.6.4 Historic and Forecasted Market Size By End User
8.6.4.1 Hospitals
8.6.4.2 Specialty Clinics
8.6.4.3 Diagnostic Centers & Others
8.6.5 Historic and Forecast Market Size by Country
8.6.5.1 Turkey
8.6.5.2 Bahrain
8.6.5.3 Kuwait
8.6.5.4 Saudi Arabia
8.6.5.5 Qatar
8.6.5.6 UAE
8.6.5.7 Israel
8.6.5.8 South Africa
8.7. South America Preeclampsia Diagnostics Market
8.7.1 Historic and Forecasted Market Size by Segments
8.7.2 Historic and Forecasted Market Size By Test type
8.7.2.1 Blood Tests
8.7.2.2 and Urine Analysis
8.7.3 Historic and Forecasted Market Size By Product
8.7.3.1 Instruments
8.7.3.2 and Consumables
8.7.4 Historic and Forecasted Market Size By End User
8.7.4.1 Hospitals
8.7.4.2 Specialty Clinics
8.7.4.3 Diagnostic Centers & Others
8.7.5 Historic and Forecast Market Size by Country
8.7.5.1 Brazil
8.7.5.2 Argentina
8.7.5.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
Q1: What would be the forecast period in the Preeclampsia Diagnostics Market research report?
A1: The forecast period in the Preeclampsia Diagnostics Market research report is 2024-2032.
Q2: Who are the key players in the Preeclampsia Diagnostics Market?
A2: F. Hoffmann-La Roche Ltd (Switzerland), PerkinElmer Inc. (Massachusetts, US), DRG INSTRUMENTS GMBH (Germany), Thermo Fisher Scientific Inc. (US), Diabetomics, Inc. (US), Metabolomic Diagnostics Ltd. (Ireland), Sera Prognostics (US), Siemens Healthineers AG (Germany), Bayer AG (Germany), Abbott Laboratories (US), Agilent Technologies, Inc. (US), Bio-Rad Laboratories, Inc. (US), Danaher Corporation (US), Illumina, Inc. (US), Natera, Inc. (US), GE Healthcare (US), Medtronic plc (Ireland), Fujifilm Holdings Corporation (Japan), Mindray Bio-Medical Electronics Co., Ltd. (China), AstraZeneca plc (UK), and Other Active Players.?
Q3: What are the segments of the Preeclampsia Diagnostics Market?
A3: The Preeclampsia Diagnostics Market is segmented into By Test type, By Product, End User and region. By Test type(Blood Tests, and Urine Analysis), By Product(Instruments, and Consumables), By End User(Hospitals, Specialty Clinics, Diagnostic Centers & Others). By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Russia, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
Q4: What is the Preeclampsia Diagnostics Market?
A4: Preeclampsia diagnostics refers to the methods and tools used to detect and monitor preeclampsia, a pregnancy-related condition characterized by high blood pressure and potential organ damage, typically occurring after the 20th week of pregnancy. Diagnostic approaches aim to identify the condition early to enable timely intervention and prevent complications for both the mother and the fetus. These methods may include blood pressure measurements, urine protein tests, and more advanced techniques such as biomarker assays that detect specific molecules linked to preeclampsia. The goal of preeclampsia diagnostics is to provide accurate, early detection, allowing healthcare providers to manage the condition effectively and improve maternal and neonatal outcomes.
Q5: How big is the Preeclampsia Diagnostics Market?
A5: Preeclampsia Diagnostics Market Size Was Valued at USD 1.1 Billion in 2023, and is Projected to Reach USD 1.4 Billion by 2032, Growing at a CAGR of 2.7% From 2024-2032.
How to Buy a Report from eminsights.jp
On the product page, choose the license you want: Single-User License, Multi-User License or Enterprise License.
If you required report in your native language, then you can click on Translated Report button and fill out the form with report name and language you want, then our team will contact you as soon as possible.
Click the Buy Now button.
You will be redirected to the checkout page. Enter your company details and payment information.
Click Place Order to complete the purchase.
Confirmation: You’ll receive an order confirmation and our team will contact you shortly with your ordered report.
If you have any questions, fill out the contact form below or email us at bizdev@eminsights.net.
Thank you for choosing eminsights.jp!