Stay Ahead in Fast-Growing Economies.
Browse Reports NowPharmacovigilance Market Size, Share, Growth & Forecast (2024-2032)
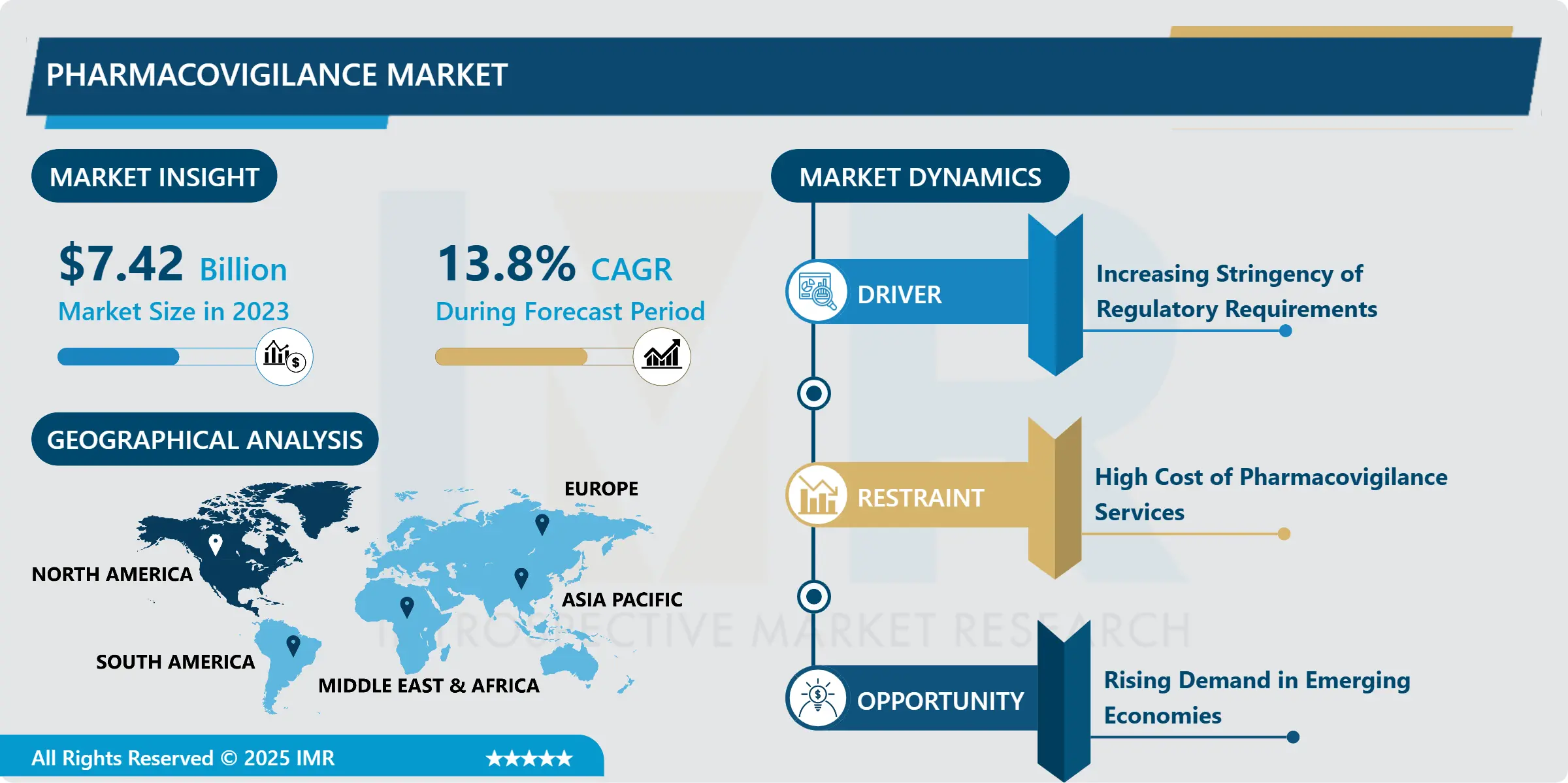
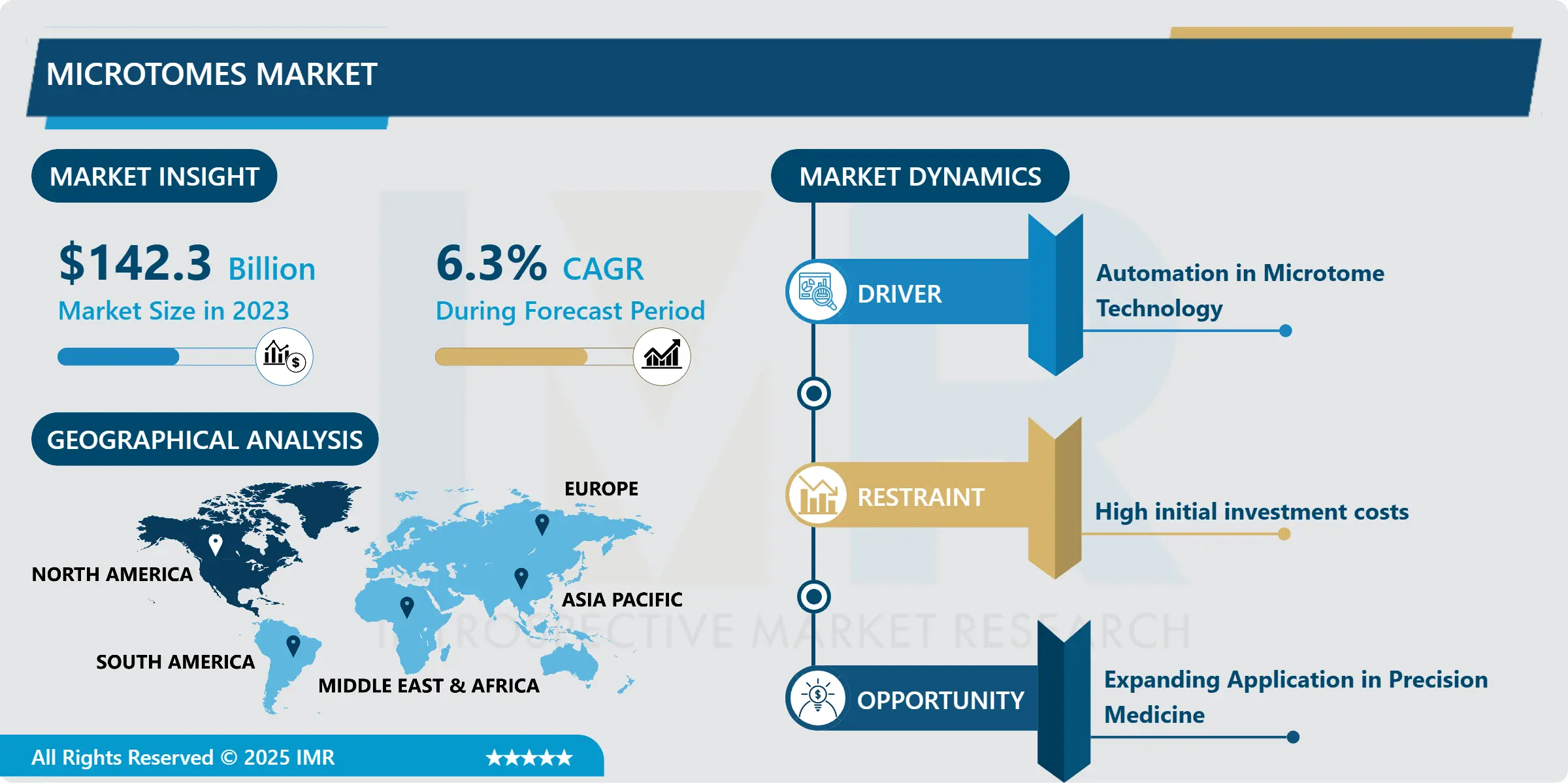
Pharmacovigilance Market Size Was Valued at USD 7.42 Billion in 2023, and is Projected to Reach USD 23.45 Billion by 2032, Growing at a CAGR of 13.8% From 2024-2032.
IMR Group
Description
Pharmacovigilance Market Synopsis
Pharmacovigilance Market Size Was Valued at USD 7.42 Billion in 2023, and is Projected to Reach USD 23.45 Billion by 2032, Growing at a CAGR of 13.8% From 2024-2032.
In this context, the pharmacovigilance market comprises of the systems, services and technologies that can be applied to the identification, evaluation, understanding and ultimately prevention of the adverse effects or any other issues related to a drug under consideration across its life cycle. It is also a crucial in controlling drug risks by allowing drug organizations and regulatory bodies to analyze risks regarding the new drug in a programmed manner. This market comprises software solutions, regulatory advisories, risk mitigation services, data management, and outsourced services for clinical trial safety data management, post-launch monitoring, and spontaneous reporting of adverse events in real-time.
The market for pharmacovigilance globally has shown a lot of growth in the recent past due to increasing rates of ADRs and increasing regulatory norms by health care authorities across the world. The fast inclination of the pharmaceutical organizations towards outsourcing pharmacovigilance services to third party vendors is the reason driving the market to augment its outsourcing services both in clinical trial and post marketing phases. Growing funding for drug safety technology like Artificial Intelligence (AI) and Machine Learning (ML) are also fuelling this segment and ensuring the more accurate and timely assessment of safety signals. Moreover, the pharmacovigilance systems in emerging economies are under development at a high rate, which will make a strong foundation for market growth.
Further, the safety concerns related to drug and increase in global awareness on drug safety along with the rising trend in patient-oriented health care solutions have fueled the need for efficient pharmacovigilance systems. The market also gains from the high growth in the related technology of pharmacovigilance software solutions to ease data acquisition, analysis, and reporting of the safety events. In addition, the growing number of cooperation between governments and healthcare systems on the formation of a single system and a single database for pharmacovigilance contributes to obtaining safer therapeutic effects. Therefore, pharmacovigilance is gradually re-positions the market towards an integrated hi- compliance analytics ecosystem that will unlock unprecedented quantitative growth drivers for stakeholders within the healthcare chain.
Pharmacovigilance Market Trend Analysis:
Integration of Artificial Intelligence (AI) in Pharmacovigilance
Pharmacovigilance has gradually experienced a shift to adopting AI and machine learning for risk assessment of drugs. Given the higher number and density of data being produced during clinical trials, as well as from subsequent use of products, the common methods of monitoring and reporting ADRs prove to be insufficient. Artificial intelligence and machine learning technologies help to avoid typical human mistakes in the work with the data and provide an opportunity to analyze big data in detail. This can help pharmacovigilance teams to identify structures in ADRs, which cannot be seen just by doing normal observations. Using AI, [the platforms] scan patient reports of all kinds and origin – documents, EHRs, and even social media to find potential new safety signals.”
When the pharmaceutical companies are committed to getting their drugs to the market faster, the solutions for pharmacovigilance become more important. Advanced intelligent-based pharmacovigilance tools do not only improve efficiency of data handling but also foster risk anticipating and managing. These technologies facilitate early safety affecting adverse events identification before they turn into major concerns. That is why AI-driven pharmacovigilance platforms are expected to become an essential component of drug development and monitoring. This trend is expected to grow at an even larger extent with AI and ML occupying the fast and central position of drug safety, compliance, and rapidly delivering new therapies to the market.
Expanding Pharmacovigilance Needs in Emerging Markets
The pharmacovigilance market has a lot of potential in emerging economies min areas like Asia-Pacific, Latin America, and Africa. The increase in the consumption of drugs within these regions that is evident is as a result of rapid economic development, the expansion of the middle class and development of improved healthcare facilities. With advancement of pharmaceuticals, these regions are coming under new pressures for the safety and reliability of drugs. In order to tackle these challenges, local governments as well as the related regulatory agencies are gradually enhancing the stringency of pharmacovigilance measures, and in doing so are often emulating the systems in Europe and North America. This trend is resulting in the increased demand for better systems of identifying safety concerns with drugs leading to more favourable opportunities for pharmacovigilance providers and the formation of particularly significant market presence in emerging markets.
Due to these shifts, there is emerging need for pharmacovigilance solutions that not only efficient, but also inexpensive and adaptable to the regulatory and health systems of these diverse countries. Pharmaceutical firm and the services vendors are searching for versatile systems that can accommodate local; regulation which differ from one country to the other. Hence, there is a great chance for unveiling new products and services to meet the needs of these markets in the light of evolving challenges. As healthcare systems evolve and regulatory bodies become more established in these emerging economies, there is potential for the pharmacovigilance service providers who have chosen these markets as key strategic markets for investment well into the future.
Pharmacovigilance Market Segment Analysis:
Pharmacovigilance Market is Segmented on the basis of Service Type, Process, End User, and Region.
By Service Type, In-house Services segment is expected to dominate the market during the forecast period
In-house Services in the pharmacovigilance market relates to the situation where any given pharmaceutical firm or healthcare delivery system centralizes its drug safety operations. This includes the creation of specific staff to manage, document, and assess ADRs besides having a way of responding to regulatory requirements. In-house pharmacovigilance hence comprises of using the company’s’ specific systems, tools and data bases on safety data. In this case, large pharma with sufficiently large budgets and internal regulatory liability prefer to implement their services in-house so that they have direct control over their safety situation and can escalate or adjust as needed on short notice in case of new safety threats.
On the other hand, Outsourced Services implies the main pharmaceutical companies and operational healthcare organizations subcontracting their drug safety monitoring and compliance to third-party vendors including CRO’s or specialized pharmacovigilance service providers. Pharmacovigilance outsourcing is rapidly increasing because of compatibility with cost savings, availability of specific skill sets, as well as overall operational flexibility in the absence of fixed internal costs. Through outsourcing, organizations can streamline their functions, while at the same time obtain competent and legally sound drug safety surveillance from outsourcing providers. This trend has however became common as the outsourcing need for pharmacovigilance services has advanced in the global pharma market.
By Process, Spontaneous Reporting segment expected to held the largest share
Spontaneous Reporting is one of the most frequently used techniques of Pharmacovigilance in which any healthcare professional, patient and other interested stakeholders report ADRs on a voluntary basis. It is a process that uses reports from the persons who have engaged in the use of or observed the harms related to drug safety. In its strength, spontaneous reporting is proficient in collecting real-world data from all over the place and hence identifying some unknown ADRs. It comes hand in hand with limited reporting as it is a passive form of surveillance where externals need to report incidents on their own.
Targeted Reporting is, therefore, a more selective form than comprehensive Reporting whereby the data of adverse events is only gathered from individual patient groups, diseases or population type, either as a response to certain risk indication or as a reaction to an emerging safety concern. Compared with spontaneous report, targeted report aims for the deliberate surveillance of specified drugs or therapeutic categories by using well-defined criteria or groups; as a result, it is more orderly and less haphazard. Literature Surveillance, another main process, involves reading and comparison of scientific literature and journals, research papers and clinical trials for reporting ADRs that may not have been reported by other means. Lastly, Active surveillance is quite an active process in which pharmacovigilance systems watch and listen to patient conditions and the status of drugs in real-time commonly by using registries, EHRs and other healthcare databases. By continuously assessing drug performance on a population basis, it is intended to pick up signs of an ADR before the problem spreads by acting as an early alert system for when remedial action is required.
Pharmacovigilance Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
North America is anticipated to retain its position as the largest region of the global pharmacovigilance market maintaining major market share in terms of market revenue. This dominance is first of all explained by the stringent regulatory standards which are set by the authorities such as FDA and HC. These agencies require that all approved drugs be backed up with extensive pharmacovigilance systems meaning that the pharmaceutical firms implement best safety measures while developing new products up to the time they are replaced by other products. Not only enforcement of the laws and regulation but high incidence of the chronic diseases caught in North America specifically in Canada and United States has encouraged the usage of the medication hence requirements of the pharmacovigilance solutions to address the patient safety regarding the ADRs.
Furthermore, the leading position of the American pharmaceutical market provides a strong incentive for pharmaceutical firms to develop and gradually implement innovative tools, including AI-based systems, for monitoring safety information rapidly increasing in volume. Such technological advancements have made it possible for healthcare institutions to conduct real-time tracking of drug safety, enhancing the time factor and precision required of possible risk determination. North America has not wavered in its promise to keep the safety of drugs their top priority, along with technological growth and the necessary protective legislation, there is no reason why North America will not continue to lead the pharmacovigilance market in the next few years.
Active Key Players in the Pharmacovigilance Market
Accenture (Ireland)
BioClinica (United States)
Capgemini (France)
Charles River Laboratories (United States)
Cognizant (United States)
Ergomed PLC (United Kingdom)
Genpact (United States)
IBM Corporation (United States)
ICON plc (Ireland)
IQVIA (United States)
PPD, Inc. (United States)
PRA Health Sciences (United States)
Wipro Ltd. (India), and Other Active Players
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter’s Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Pharmacovigilance Market by Type
4.1 Pharmacovigilance Market Snapshot and Growth Engine
4.2 Pharmacovigilance Market Overview
4.3 In-house Services
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 In-house Services: Geographic Segmentation Analysis
4.4 Outsourced Services
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 Outsourced Services: Geographic Segmentation Analysis
Chapter 5: Pharmacovigilance Market by Application
5.1 Pharmacovigilance Market Snapshot and Growth Engine
5.2 Pharmacovigilance Market Overview
5.3 Spontaneous Reporting
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Spontaneous Reporting: Geographic Segmentation Analysis
5.4 Targeted Reporting
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.4.3 Key Market Trends, Growth Factors and Opportunities
5.4.4 Targeted Reporting: Geographic Segmentation Analysis
5.5 Literature Surveillance
5.5.1 Introduction and Market Overview
5.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.5.3 Key Market Trends, Growth Factors and Opportunities
5.5.4 Literature Surveillance: Geographic Segmentation Analysis
5.6 Active Surveillance
5.6.1 Introduction and Market Overview
5.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.6.3 Key Market Trends, Growth Factors and Opportunities
5.6.4 Active Surveillance: Geographic Segmentation Analysis
Chapter 6: Pharmacovigilance Market by End User
6.1 Pharmacovigilance Market Snapshot and Growth Engine
6.2 Pharmacovigilance Market Overview
6.3 Pharmaceutical Companies
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.3.3 Key Market Trends, Growth Factors and Opportunities
6.3.4 Pharmaceutical Companies: Geographic Segmentation Analysis
6.4 Contract Research Organizations (CROs)
6.4.1 Introduction and Market Overview
6.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.4.3 Key Market Trends, Growth Factors and Opportunities
6.4.4 Contract Research Organizations (CROs): Geographic Segmentation Analysis
6.5 Biopharmaceutical Companies
6.5.1 Introduction and Market Overview
6.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.5.3 Key Market Trends, Growth Factors and Opportunities
6.5.4 Biopharmaceutical Companies: Geographic Segmentation Analysis
6.6 Other Healthcare Providers
6.6.1 Introduction and Market Overview
6.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.6.3 Key Market Trends, Growth Factors and Opportunities
6.6.4 Other Healthcare Providers: Geographic Segmentation Analysis
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Pharmacovigilance Market Share by Manufacturer (2023)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 ACCENTURE (IRELAND)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 BIOCLINICA (UNITED STATES)
7.4 CAPGEMINI (FRANCE)
7.5 CHARLES RIVER LABORATORIES (UNITED STATES)
7.6 COGNIZANT (UNITED STATES)
7.7 ERGOMED PLC (UNITED KINGDOM)
7.8 GENPACT (UNITED STATES)
7.9 IBM CORPORATION (UNITED STATES)
7.10 ICON PLC (IRELAND)
7.11 IQVIA (UNITED STATES)
7.12 PPD INC. (UNITED STATES)
7.13 PRA HEALTH SCIENCES (UNITED STATES)
7.14 WIPRO LTD. (INDIA)
7.15 OTHER ACTIVE PLAYERS
Chapter 8: Global Pharmacovigilance Market By Region
8.1 Overview
8.2. North America Pharmacovigilance Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By Type
8.2.4.1 In-house Services
8.2.4.2 Outsourced Services
8.2.5 Historic and Forecasted Market Size By Application
8.2.5.1 Spontaneous Reporting
8.2.5.2 Targeted Reporting
8.2.5.3 Literature Surveillance
8.2.5.4 Active Surveillance
8.2.6 Historic and Forecasted Market Size By End User
8.2.6.1 Pharmaceutical Companies
8.2.6.2 Contract Research Organizations (CROs)
8.2.6.3 Biopharmaceutical Companies
8.2.6.4 Other Healthcare Providers
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Pharmacovigilance Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By Type
8.3.4.1 In-house Services
8.3.4.2 Outsourced Services
8.3.5 Historic and Forecasted Market Size By Application
8.3.5.1 Spontaneous Reporting
8.3.5.2 Targeted Reporting
8.3.5.3 Literature Surveillance
8.3.5.4 Active Surveillance
8.3.6 Historic and Forecasted Market Size By End User
8.3.6.1 Pharmaceutical Companies
8.3.6.2 Contract Research Organizations (CROs)
8.3.6.3 Biopharmaceutical Companies
8.3.6.4 Other Healthcare Providers
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Pharmacovigilance Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By Type
8.4.4.1 In-house Services
8.4.4.2 Outsourced Services
8.4.5 Historic and Forecasted Market Size By Application
8.4.5.1 Spontaneous Reporting
8.4.5.2 Targeted Reporting
8.4.5.3 Literature Surveillance
8.4.5.4 Active Surveillance
8.4.6 Historic and Forecasted Market Size By End User
8.4.6.1 Pharmaceutical Companies
8.4.6.2 Contract Research Organizations (CROs)
8.4.6.3 Biopharmaceutical Companies
8.4.6.4 Other Healthcare Providers
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Pharmacovigilance Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By Type
8.5.4.1 In-house Services
8.5.4.2 Outsourced Services
8.5.5 Historic and Forecasted Market Size By Application
8.5.5.1 Spontaneous Reporting
8.5.5.2 Targeted Reporting
8.5.5.3 Literature Surveillance
8.5.5.4 Active Surveillance
8.5.6 Historic and Forecasted Market Size By End User
8.5.6.1 Pharmaceutical Companies
8.5.6.2 Contract Research Organizations (CROs)
8.5.6.3 Biopharmaceutical Companies
8.5.6.4 Other Healthcare Providers
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Pharmacovigilance Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By Type
8.6.4.1 In-house Services
8.6.4.2 Outsourced Services
8.6.5 Historic and Forecasted Market Size By Application
8.6.5.1 Spontaneous Reporting
8.6.5.2 Targeted Reporting
8.6.5.3 Literature Surveillance
8.6.5.4 Active Surveillance
8.6.6 Historic and Forecasted Market Size By End User
8.6.6.1 Pharmaceutical Companies
8.6.6.2 Contract Research Organizations (CROs)
8.6.6.3 Biopharmaceutical Companies
8.6.6.4 Other Healthcare Providers
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Pharmacovigilance Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By Type
8.7.4.1 In-house Services
8.7.4.2 Outsourced Services
8.7.5 Historic and Forecasted Market Size By Application
8.7.5.1 Spontaneous Reporting
8.7.5.2 Targeted Reporting
8.7.5.3 Literature Surveillance
8.7.5.4 Active Surveillance
8.7.6 Historic and Forecasted Market Size By End User
8.7.6.1 Pharmaceutical Companies
8.7.6.2 Contract Research Organizations (CROs)
8.7.6.3 Biopharmaceutical Companies
8.7.6.4 Other Healthcare Providers
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
Q1: What would be the forecast period in the Pharmacovigilance Market research report?
A1: The forecast period in the Pharmacovigilance Market research report is 2024-2032.
Q2: Who are the key players in the Pharmacovigilance Market?
A2: Accenture, BioClinica, Charles River Laboratories, Cognizant, and IBM Corporation. and Other Major Players.
Q3: What are the segments of the Pharmacovigilance Market?
A3: The Pharmacovigilance Market is segmented into Service Type, Process, End User, By Solution, and region. By Service Type, the market is categorized into In-house Services, Outsourced Services. By Process, the market is categorized into Spontaneous Reporting, Targeted Reporting, Literature Surveillance, and Active Surveillance. By End User, the market is categorized into Pharmaceutical Companies, Contract Research Organizations (CROs), Biopharmaceutical Companies, and Others. By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
Q4: What is the Pharmacovigilance Market?
A4: In this context, the pharmacovigilance market comprises of the systems, services and technologies that can be applied to the identification, evaluation, understanding and ultimately prevention of the adverse effects or any other issues related to a drug under consideration across its life cycle. It is also a crucial in controlling drug risks by allowing drug organizations and regulatory bodies to analyze risks regarding the new drug in a programmed manner. This market comprises software solutions, regulatory advisories, risk mitigation services, data management, and outsourced services for clinical trial safety data management, post-launch monitoring, and spontaneous reporting of adverse events in real-time.
Q5: How big is the Pharmacovigilance Market?
A5: Pharmacovigilance Market Size Was Valued at USD 7.42 Billion in 2023, and is Projected to Reach USD 23.45 Billion by 2032, Growing at a CAGR of 13.8% From 2024-2032.
How to Buy a Report from eminsights.jp
On the product page, choose the license you want: Single-User License, Multi-User License or Enterprise License.
If you required report in your native language, then you can click on Translated Report button and fill out the form with report name and language you want, then our team will contact you as soon as possible.
Click the Buy Now button.
You will be redirected to the checkout page. Enter your company details and payment information.
Click Place Order to complete the purchase.
Confirmation: You’ll receive an order confirmation and our team will contact you shortly with your ordered report.
If you have any questions, fill out the contact form below or email us at bizdev@eminsights.net.
Thank you for choosing eminsights.jp!