Stay Ahead in Fast-Growing Economies.
Browse Reports NowPharma 4.0 Market Size, Trends, and Forecast Analysis (2024-2032)
Industry 4.0 for pharma contains smart automation, digitalization and data exchange and transmission system inside the pharma industry known as Pharma 4.0.
IMR Group
Description
Pharma 4.0 Market Synopsis:
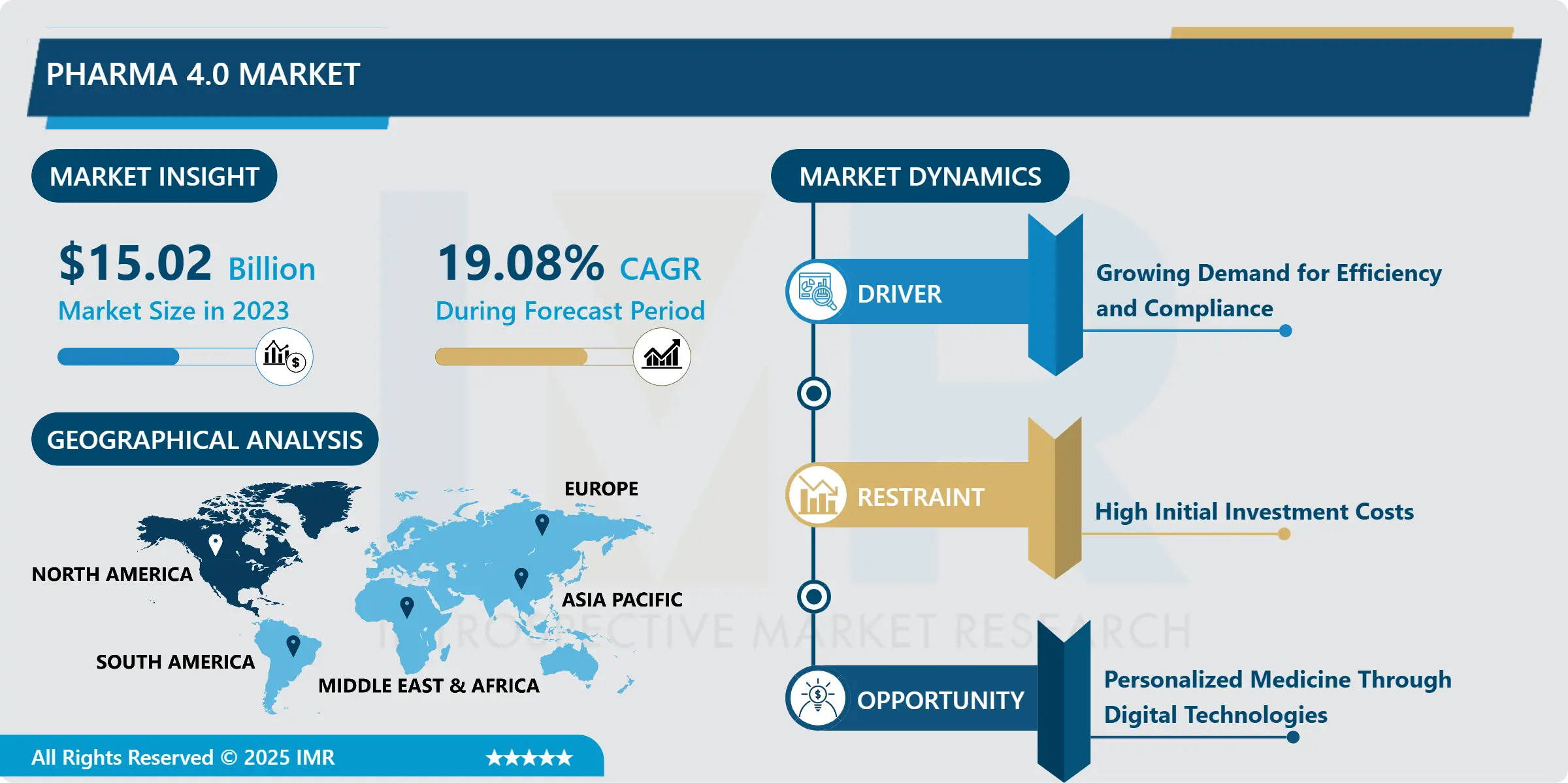
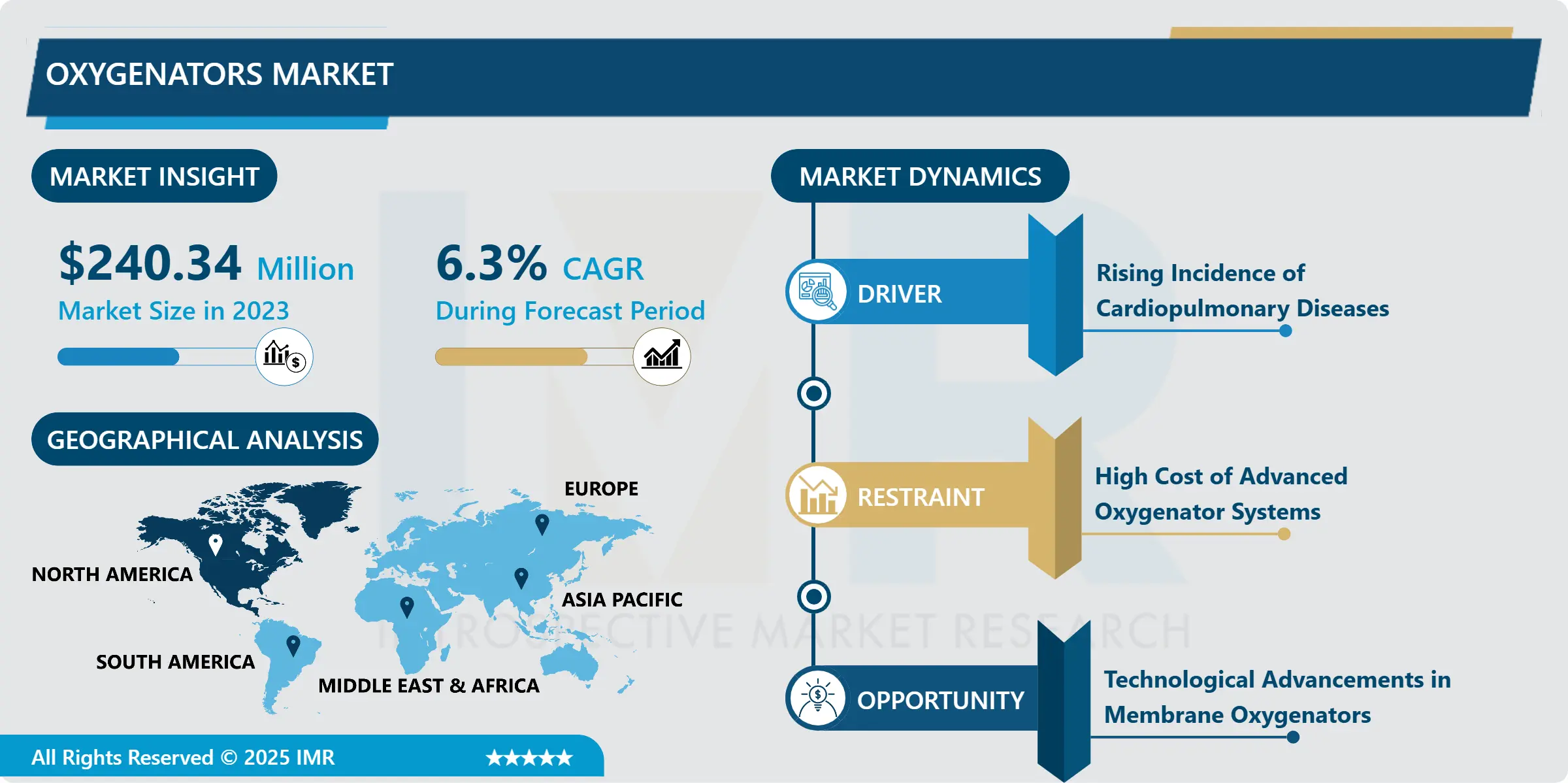
Pharma 4.0 Market Size Was Valued at USD 15.02 Billion in 2023, and is Projected to Reach USD 72.31 Billion by 2032, Growing at a CAGR of 19.08% From 2024-2032.
Industry 4.0 for pharma contains smart automation, digitalization and data exchange and transmission system inside the pharma industry known as Pharma 4.0. This car focuses on acting as a concept that entails the use of new trends such as the internet of things, artificial intelligence, cloud computing and big data in the functioning of pharmaceutical businesses. The purpose is to drive the manufacturing incrementally, development of new drugs and agency compliances through better work flow, better quality control as well better work efficiency.
Indeed, the market known as Pharma 4.0 brings about change in the environment within the pharmaceutical industry due to implementation of fertile technologies and digital technology innovation. The following changes are witnessed due to advantages associated with the need for efficiency, compliance to regulatory frameworks, and the increased promotion of personalized medicine. Pharma 4.0 means the continuation of the industry 4.0 which is focused on agile manufacturing with real-time monitoring and computational processing of data for prediction of various results. Some areas of benefit include enhancing the automation of production processes, enhancement of supply chain processes, and acquiring enhanced capacity to respond effectively to the market needs depending on enhanced decision making as well as personalized data analysis.
Introduction to Pharma 4.0 is informed by the increasing need to meet challenges like high production cost, lengthy drug production, and rigid regulatory mechanisms. Such measures do not only facilitate operations but they also monitor the chain un bounded to ensure product quality and safety. Implementations of cloud enable an organization to foster efficient networks of global value chain involving the pharmaceutical companies, and allow visibility of compliance at the value chain. This approach can also be attributed to the creation of Pharma 4 and the need for the creation of precision medicine, which are more flexible in production.
Pharma 4.0 Market Trend Analysis:
Integration of Artificial Intelligence in Drug Development
Two of the most apparent trends in the Pharma 4.0 market are the advanced integration of AI in drug development. Many pharmaceutical firms are now increasingly using artificial intelligence algorithms to speed up the drug discovery process to ensure they establish probable drug candidates much faster. AI makes it easier to understand big data, analyse drug interactions and shape clinical trials in a way that minimizes the overall time to market. For instance, AI can look through billions of compounds within a short span of time and the researchers can look at the probabilities. This not only saves costs but increases the chances of finding cure to such complicated diseases as well as treatments to accompany them.
Considering the increasing application of AI in drug discovery, the paradigm of pharmacological and medical research is changing. This means that while point startups and large players are engaging with tech firms to boost the chances of AI predicting drug efficacy and safety. This trend is also envisaged to advance as AI progresses, to provide comprehensive understanding of molecular biology, patient’s prognosis, and individualized treatment. Therefore, the use of AI in predictive modelling and simulation is also gaining importance for the regulatory submission where AI insights are helping in speeding up the approval process.
Personalized Medicine Through Digital Technologies
Another of the most significant chances which can be seen in the frame of the Pharma 4.0 market is individual approach. With the use of big data analytics, AI, and IoT, pharmaceutical companies are capable of accelerating ways of creating drugs that especially befit specific patient choices. Ultra personalised medicine means that treatments are tailored to an individual’s genetic profile and other characteristics, so the patient has a greater chance of achieving a favourable result. Pharma 4.0 helps to gather data to develop these treatments and gives healthcare workers immediate feedback from patients.
This opportunity is especially important in the area of oncology, a unique therapy where long-term positive outcomes for tumour survival and lowered side effects have been noted. Integrating Pharma 4.0 technologies into organizations allows the delivery of more effective therapies with lesser trial and error approaches hence increasing efficient ways of health care delivery hence lowering the costs of health care.
Pharma 4.0 Market Segment Analysis:
Pharma 4.0 Market is Segmented on the basis of Technology, Application, End User, and Region.
By Technology, Internet of Things (IoT) segment is expected to dominate the market during the forecast period
The emerging technologies that are in use in the Pharma 4.0 market are cloud computing, AI, and big data analytics as well as the IoT. cloud computing helps pharma companies to manage their large data securely and effectively at lesser cost. It helps to co-ordinate members working in different team or in different geographical location; thus, it enhances the level of co- ordination in organizations. AI is being rapidly applied across the drug development process, including compound screening, patient monitoring in clinical trials, and predictive modelling. Ultimately, AI brings to the process better perspectives of the patient data, disease profile and even the possible effectiveness of a treatment plan.
As big data continues to flood different sectors, an important function it serves is at for interpreting data accumulated from different phases of the drug development and production process. Using data collected from clinical trials, manufacturing plants and patient feedback means that companies make better decisions and operate more efficiently. IoT can be used in tracking equipment’s, manufacturing facilities and patients’ health among others which will improve on productivity and quality of products. Altogether these technologies are recognized as Pharma 4.0, as the key source of innovation and competitive advantage.
By Application, Clinical Trials segment expected to held the largest share
Technologies linked to Pharma 4.0 are being deployed at different levels ranging from discovery and development of drugs right to clinical trials and manufacturing. In drug discovery, thru the use of hi-tech tools such as AI and big data analytics, druggist can quickly come up with drug leads hence fast development cycles and lesser cost on research. The former can imitate living object communication and seize the drug effects on the body, enhancing precision during the preclinical stage. Moreover, through technology-driven clinical management systems, Pharma 4.0 is also successfully undertaking clinical trials that are holistic and easy to recruit and monitor, and also gather information and data that is in compliance to the rules.
Manufacturing is the second focal sector where the concept of Pharma 4.0 is creating a strong revelation. Computerized technologies minimize the presence of a human being in the production process while at the same time improving efficiency and quality. IoT and AI have been adopted to track the working conditions of equipment’s and timely diagnose any component failure in order to minimize production loss in the manufacturing processes. This integration is enabling the industry to produce drugs more sensitively to the market demand, flexible and sustainable in a way.
Pharma 4.0 Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
The Pharma 4.0 market is dominated by North America because of the highly developed technological base and significant number of large pharmaceutical companies. It has been noted that the region is significantly adopting Industry 4.0 principles and its pharmaceutical industry benefits from well-developed rules and proper regulatory environment that promote the further use of high technologies to guarantee the quality and compliance of products. Also, owing to the U.S government highlight on healthcare innovation and subsequent funding on technologies in digital health have advanced the implementation of Pharma 4.0 solutions in the region.
Notably, the United States is host to many pharmaceutical and biotechnology firms who are already using AI, IoTs, and big data in order to ‘discovery/develop new drugs and create better manufacturing processes. Pharmaceutical and technology companies are in close partnership working on solutions that help automate, oPtimise or enhance workflow, and ultimately bring better patient care. Taking into account the increasing popularity of the field of Pharma 4.0, North America should maintain its leading position in the market for personalized medicine in the future.
Active Key Players in the Pharma 4.0 Market
Pfizer (USA)
Roche (Switzerland)
Johnson & Johnson (USA)
Novartis (Switzerland)
Merck & Co. (USA)
GlaxoSmithKline (UK)
Sanofi (France)
AstraZeneca (UK)
AbbVie (USA)
Eli Lilly (USA)
Bayer (Germany)
Bristol-Myers Squibb (USA), and Other Active Players
Key Industry Developments in the Pharma 4.0 Market:
In May 2024, Sanofi has collaborated with Formation Bio and OpenAI to harness AI to expedite drug development. To streamline the process of bringing new medicines to patients, the companies will combine their data, software and tuned models to create purpose-built solutions across the development lifecycle of the drugs.
In April 2024, Avery Dennison has partnered with Controlant, one of the leaders in digital transformation of pharma supply chains, to drive real-time, end-to-end visibility and support sustainability initiatives in the pharma industry.
In August 2023, Solve. carelaunched a novel Care.Trials Network. This cutting-edge network, backed by blockchain and Zero-knowledge (ZK) technology, offered novel solutions to conduct clinical trials.
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter’s Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Pharma 4.0 Market by Technology
4.1 Pharma 4.0 Market Snapshot and Growth Engine
4.2 Pharma 4.0 Market Overview
4.3 Cloud Computing
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 Cloud Computing: Geographic Segmentation Analysis
4.4 Artificial Intelligence (AI)
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 Artificial Intelligence (AI): Geographic Segmentation Analysis
4.5 Big Data Analytics
4.5.1 Introduction and Market Overview
4.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.5.3 Key Market Trends, Growth Factors and Opportunities
4.5.4 Big Data Analytics: Geographic Segmentation Analysis
4.6 Internet of Things (IoT)
4.6.1 Introduction and Market Overview
4.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.6.3 Key Market Trends, Growth Factors and Opportunities
4.6.4 Internet of Things (IoT): Geographic Segmentation Analysis
Chapter 5: Pharma 4.0 Market by Application
5.1 Pharma 4.0 Market Snapshot and Growth Engine
5.2 Pharma 4.0 Market Overview
5.3 Drug Discovery and Development
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Drug Discovery and Development: Geographic Segmentation Analysis
5.4 Clinical Trials
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.4.3 Key Market Trends, Growth Factors and Opportunities
5.4.4 Clinical Trials: Geographic Segmentation Analysis
5.5 Manufacturing)
5.5.1 Introduction and Market Overview
5.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.5.3 Key Market Trends, Growth Factors and Opportunities
5.5.4 Manufacturing): Geographic Segmentation Analysis
Chapter 6: Pharma 4.0 Market by End-User
6.1 Pharma 4.0 Market Snapshot and Growth Engine
6.2 Pharma 4.0 Market Overview
6.3 Pharmaceutical Companies
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.3.3 Key Market Trends, Growth Factors and Opportunities
6.3.4 Pharmaceutical Companies: Geographic Segmentation Analysis
6.4 Biotechnology Companies
6.4.1 Introduction and Market Overview
6.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.4.3 Key Market Trends, Growth Factors and Opportunities
6.4.4 Biotechnology Companies: Geographic Segmentation Analysis
6.5 CROs and CMOs
6.5.1 Introduction and Market Overview
6.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.5.3 Key Market Trends, Growth Factors and Opportunities
6.5.4 CROs and CMOs: Geographic Segmentation Analysis
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Pharma 4.0 Market Share by Manufacturer (2023)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 PFIZER (USA)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 ROCHE (SWITZERLAND)
7.4 JOHNSON & JOHNSON (USA)
7.5 NOVARTIS (SWITZERLAND)
7.6 MERCK & CO. (USA)
7.7 GLAXOSMITHKLINE (UK)
7.8 SANOFI (FRANCE)
7.9 ASTRAZENECA (UK)
7.10 ABBVIE (USA)
7.11 ELI LILLY (USA)
7.12 BAYER (GERMANY)
7.13 BRISTOL-MYERS SQUIBB (USA)
7.14 OTHER ACTIVE PLAYERS
Chapter 8: Global Pharma 4.0 Market By Region
8.1 Overview
8.2. North America Pharma 4.0 Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By Technology
8.2.4.1 Cloud Computing
8.2.4.2 Artificial Intelligence (AI)
8.2.4.3 Big Data Analytics
8.2.4.4 Internet of Things (IoT)
8.2.5 Historic and Forecasted Market Size By Application
8.2.5.1 Drug Discovery and Development
8.2.5.2 Clinical Trials
8.2.5.3 Manufacturing)
8.2.6 Historic and Forecasted Market Size By End-User
8.2.6.1 Pharmaceutical Companies
8.2.6.2 Biotechnology Companies
8.2.6.3 CROs and CMOs
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Pharma 4.0 Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By Technology
8.3.4.1 Cloud Computing
8.3.4.2 Artificial Intelligence (AI)
8.3.4.3 Big Data Analytics
8.3.4.4 Internet of Things (IoT)
8.3.5 Historic and Forecasted Market Size By Application
8.3.5.1 Drug Discovery and Development
8.3.5.2 Clinical Trials
8.3.5.3 Manufacturing)
8.3.6 Historic and Forecasted Market Size By End-User
8.3.6.1 Pharmaceutical Companies
8.3.6.2 Biotechnology Companies
8.3.6.3 CROs and CMOs
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Pharma 4.0 Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By Technology
8.4.4.1 Cloud Computing
8.4.4.2 Artificial Intelligence (AI)
8.4.4.3 Big Data Analytics
8.4.4.4 Internet of Things (IoT)
8.4.5 Historic and Forecasted Market Size By Application
8.4.5.1 Drug Discovery and Development
8.4.5.2 Clinical Trials
8.4.5.3 Manufacturing)
8.4.6 Historic and Forecasted Market Size By End-User
8.4.6.1 Pharmaceutical Companies
8.4.6.2 Biotechnology Companies
8.4.6.3 CROs and CMOs
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Pharma 4.0 Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By Technology
8.5.4.1 Cloud Computing
8.5.4.2 Artificial Intelligence (AI)
8.5.4.3 Big Data Analytics
8.5.4.4 Internet of Things (IoT)
8.5.5 Historic and Forecasted Market Size By Application
8.5.5.1 Drug Discovery and Development
8.5.5.2 Clinical Trials
8.5.5.3 Manufacturing)
8.5.6 Historic and Forecasted Market Size By End-User
8.5.6.1 Pharmaceutical Companies
8.5.6.2 Biotechnology Companies
8.5.6.3 CROs and CMOs
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Pharma 4.0 Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By Technology
8.6.4.1 Cloud Computing
8.6.4.2 Artificial Intelligence (AI)
8.6.4.3 Big Data Analytics
8.6.4.4 Internet of Things (IoT)
8.6.5 Historic and Forecasted Market Size By Application
8.6.5.1 Drug Discovery and Development
8.6.5.2 Clinical Trials
8.6.5.3 Manufacturing)
8.6.6 Historic and Forecasted Market Size By End-User
8.6.6.1 Pharmaceutical Companies
8.6.6.2 Biotechnology Companies
8.6.6.3 CROs and CMOs
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Pharma 4.0 Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By Technology
8.7.4.1 Cloud Computing
8.7.4.2 Artificial Intelligence (AI)
8.7.4.3 Big Data Analytics
8.7.4.4 Internet of Things (IoT)
8.7.5 Historic and Forecasted Market Size By Application
8.7.5.1 Drug Discovery and Development
8.7.5.2 Clinical Trials
8.7.5.3 Manufacturing)
8.7.6 Historic and Forecasted Market Size By End-User
8.7.6.1 Pharmaceutical Companies
8.7.6.2 Biotechnology Companies
8.7.6.3 CROs and CMOs
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
Q1: What would be the forecast period in the Pharma 4.0 Market research report?
A1: The forecast period in the Pharma 4.0 Market research report is 2024-2032.
Q2: Who are the key players in the Pharma 4.0 Market?
A2: Pfizer (USA), Roche (Switzerland), Johnson & Johnson (USA), Novartis (Switzerland), Merck & Co. (USA), GlaxoSmithKline (UK), Sanofi (France), AstraZeneca (UK), AbbVie (USA), Eli Lilly (USA), Bayer (Germany), Bristol-Myers Squibb (USA), and Other Active Players.
Q3: What are the segments of the Pharma 4.0 Market?
A3: The Pharma 4.0 Market is segmented into Technology, Application, End User, and Region. By Technology, the market is categorized into Cloud Computing, Artificial Intelligence (AI), Big Data Analytics, and Internet of Things (IoT). By Application, the market is categorized into Drug Discovery and Development, Clinical Trials, and Manufacturing. By End-User, the market is categorized into Pharmaceutical Companies, Biotechnology Companies, CROs, and CMOs. By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
Q4: What is the Pharma 4.0 Market?
A4: Industry 4.0 for pharma contains smart automation, digitalization, and data exchange and transmission system inside the pharma industry known as Pharma 4.0. This car focuses on acting as a concept that entails the use of new trends such as the internet of things, artificial intelligence, cloud computing and big data in the functioning of pharmaceutical businesses. The purpose is to drive the manufacturing incrementally, development of new drugs and agency compliances through better work flow, better quality control as well better work efficiency.
Q5: How big is the Pharma 4.0 Market?
A5: Pharma 4.0 Market Size Was Valued at USD 15.02 Billion in 2023, and is Projected to Reach USD 72.31 Billion by 2032, Growing at a CAGR of 19.08% From 2024-2032.
How to Buy a Report from eminsights.jp
On the product page, choose the license you want: Single-User License, Multi-User License or Enterprise License.
If you required report in your native language, then you can click on Translated Report button and fill out the form with report name and language you want, then our team will contact you as soon as possible.
Click the Buy Now button.
You will be redirected to the checkout page. Enter your company details and payment information.
Click Place Order to complete the purchase.
Confirmation: You’ll receive an order confirmation and our team will contact you shortly with your ordered report.
If you have any questions, fill out the contact form below or email us at bizdev@eminsights.net.
Thank you for choosing eminsights.jp!