Stay Ahead in Fast-Growing Economies.
Browse Reports NowCompanion Diagnostics Market – Trends, Size & Outlook (2024-2032)
Companion diagnostics refers to diagnostic tests that are used in determining the presence of biomarkers in the patents for efficient decision making on the best treatment to use especially the molecular basis of many diseases today is being established. Companion diagnostics are most commonly used in oncology as they are vital for choosing patients who likely will benefit from some of the targeted treatments due to the genetic profile of the tumor. These tests are gradually becoming incorporated into patient’s management plans to make sure that the therapies that are given to patients are accorded to their specific genetic makeup, making the treatment more effective while reducing the side effects.
IMR Group
Description
Companion Diagnostics Market Synopsis:
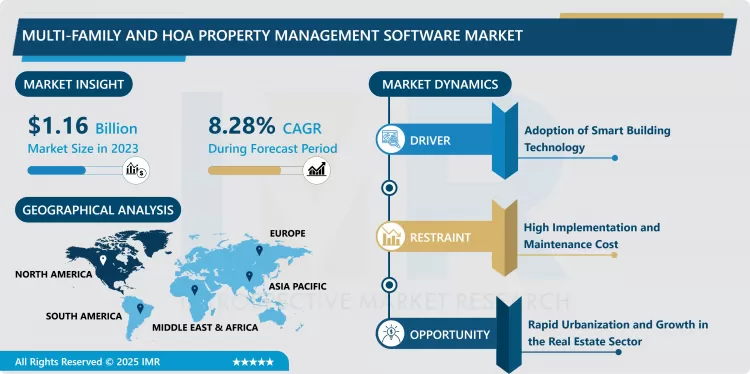
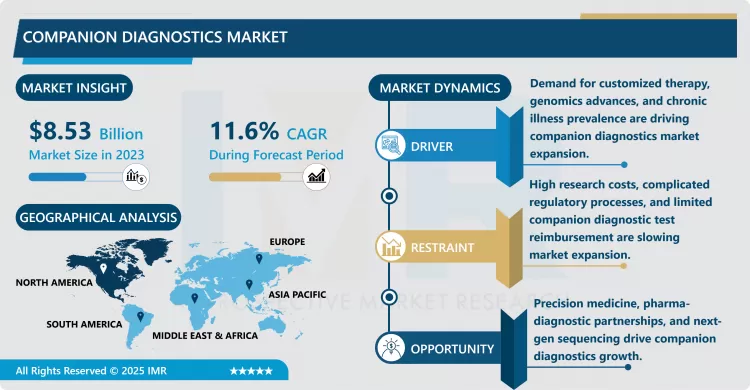
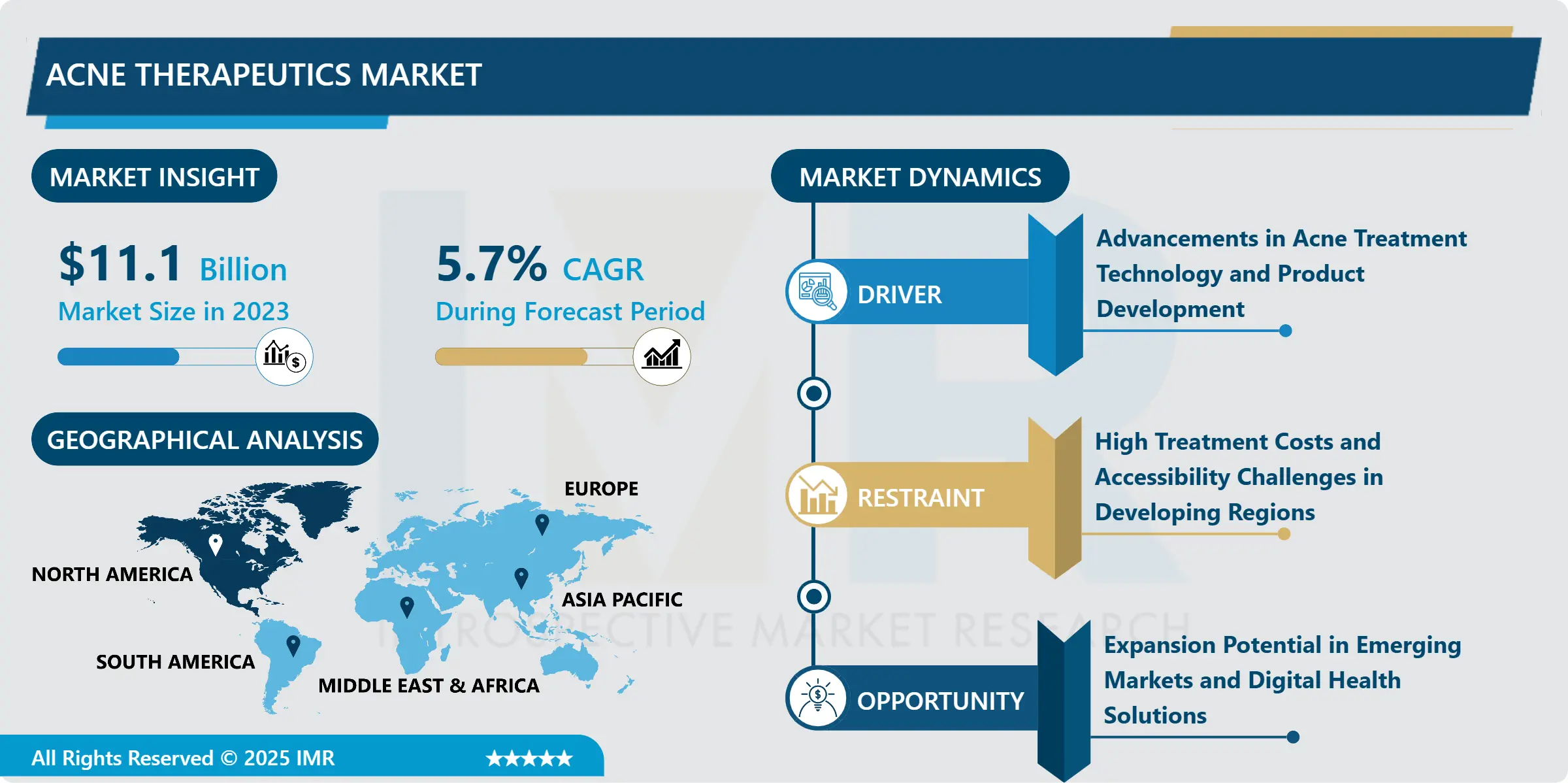
Companion Diagnostics Market Size Was Valued at USD 8.53 Billion in 2023, and is Projected to Reach USD 22.90 Billion by 2032, Growing at a CAGR of 11.6% From 2024-2032.
Companion diagnostics refers to diagnostic tests that are used in determining the presence of biomarkers in the patents for efficient decision making on the best treatment to use especially the molecular basis of many diseases today is being established. Companion diagnostics are most commonly used in oncology as they are vital for choosing patients who likely will benefit from some of the targeted treatments due to the genetic profile of the tumor. These tests are gradually becoming incorporated into patient’s management plans to make sure that the therapies that are given to patients are accorded to their specific genetic makeup, making the treatment more effective while reducing the side effects.
The main reasons for the development of the companion diagnostics market include increased incidence of chronic diseases, especially cancer, as well as the development of genomics and biotechnology.. With a various number of drugs developed for specific molecular targets, there is increasing demand for diagnostics to accompany these drugs as they would not benefit all patients. The regulators including the FDA are also encouraging growth of this market through early clearance of companion diagnostics with prove safety and efficacy. These have pigeonhole the diagnostic companies and drug makers closer together, thus fastering the delivery of new diagnostics and therapeutics.• The growth of the markets for companion diagnostics is also expected thanks to the development of next generation sequencing (NGS) and other high throughout techniques, as they provide quick and full genetic analysis.rugs, there is a growing need for companion diagnostics to ensure these treatments are provided to the right patients. Regulatory agencies, such as the FDA, are also supporting the growth of this market by fast-tracking approvals for companion diagnostics that demonstrate safety and efficacy. This has led to increased collaboration between diagnostic companies and drug manufacturers, accelerating the availability of new diagnostics and therapeutics.
Opportunities in the companion diagnostics market are expanding with the development of next-generation sequencing (NGS) and other high-throughput technologies that allow for rapid and comprehensive genetic testing. It is evident from the present analysis that these emerging markets, which have inadequate but enhancing health care systems, and an increasing appreciation of precision medicine to harness their boundless growth possibilities. However, there are also problems such as high development costs, high regulatory requirements and a large amount of clinical testing, which affect the entry of new diagnostic solutions. However, with healthcare being increasingly oriented toward individual needs, the companion diagnostics market is becoming more popular.
Companion Diagnostics Market Trend Analysis:
Expansion of Next-Generation Sequencing (NGS) in Companion Diagnostics
Present-day companion diagnostics are also being driven by next-generation sequencing (NGS), The NGS is more accurate, cheaper, and faster than traditional technologies that can give genomic profiles of multiple biomarkers at once.. This capability is particularly significant in oncology because a single NGS test can offer a vast amount of information about the tumor, including genetic mutations, to inform improved treatment plans. The innovation of new NGS technology has also spurred the need for multi-gene panel companion diagnostics across the globe as part of personalized medicine This has further boosted growth of the market and better patient treatment outcomes.
Growing Pharmaceutical-Diagnostic Partnerships
Companion diagnostics are in high demand and companies in the pharma and diagnostic sectors see strategic value of combined development of targeted therapies and their diagnostic tests. These collaborations guarantee that drugs are aligned to accurate diagnostic equipment resulting in enhanced treatment outcome and a higher pass rate on FDA’s approval dial. This trend has created many cooperative ventures thus enabling quicker development of companion diagnostics that address the needs of the particular therapy, most commonly associated with the diseases that fall under oncological, cardiovascular, and immunological classes, which has widened the market’s purview.
Companion Diagnostics Market Segment Analysis:
Companion Diagnostics Market is Segmented on the basis of Product and Service, Technology, Indication, End-User, and Region
By Product And Service, Kits and Reagents segment is expected to dominate the market during the forecast period
In the companion diagnostics market, some products and services can be differentiated as kits and reagents and software & services; both are essential in delivering accurate diagnostic tools.. Reagents, kits, and biochemical are vital to the laboratory to carry out tests and provide diagnostic information required to assess modifications, biomarkers, and other genetic markers as the basis for approved targeted therapies. However, there exists software and services that assist in product analysis of this kind, help in aggregating data from electronic health records, and help in the interpretation of the results, which usually is an important component in the clinical decision-making process. The need for better, more precise and timely patient data processing, especially in individualized medicine, is pushing advances as well as investments in both types of product.
By End-User, Reference Laboratories segment expected to held the largest share
In the companion diagnostics market, major customers are the reference laboratories and players from the pharmaceutical and biopharmaceutical industries.. Reference laboratories work for diagnosis to ensure biomarkers to drive treatment options mainly in oncology and rare diseases. These labs are very important in making sure that the diagnostic tools to be used in the diagnostic centers are very accurate. Biotech and pharmaceutical industries are also major participants here because they often work hand in hand with diagnostic companies that work to create companion diagnostics that track with particular therapies. These affiliations assist in speeding up the process through which new drugs gain approval from regulatory authorities and assist in delivering treatments that best serve the patient population to improve results of treatment, and increase progress toward personalized medicine.
Companion Diagnostics Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
North America is anticipated to be the largest market for companion diagnostics from 2017 to 2022 due to factors such as developed healthcare sector, high spending on the health care sector, and growing trend for personalized medicine.. The population takes a lot of benefits of tender, especially focused on oncology and genetic researches and, therefore, application of companion diagnostics.. Furthermore, Americas has seen innovative support from agencies such as the FDA on how to fast-track and commercialize companion diagnostics thus enhancing the market. Largely due to the presence of large pharmaceutical and diagnostic companies, and an up-tick in patients demanding personalised drugs, the companion diagnostics market in North America continues to grow.
Active Key Players in the Companion Diagnostics Market:
Abbott (USA), Agilent Technologies Inc. (USA)
F. Hoffmann-La Roche Ltd. (Switzerland)
Guardant Health (USA)
QIAGEN (Germany)
Myriad Genetics, Inc. (USA)
Illumina Inc. (USA)
Thermo Fisher Scientific Inc. (USA)
BIOMERIEUX (France)
Myriad Genetics Inc. (USA)
Other Active Players.
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter’s Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Companion Diagnostics Market by Product And Service (2018-2032)
4.1 Companion Diagnostics Market Snapshot and Growth Engine
4.2 Market Overview
4.3 Kits And Reagents
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
4.3.3 Key Market Trends, Growth Factors, and Opportunities
4.3.4 Geographic Segmentation Analysis
4.4 Software And Services
Chapter 5: Companion Diagnostics Market by Technology (2018-2032)
5.1 Companion Diagnostics Market Snapshot and Growth Engine
5.2 Market Overview
5.3 Polymerase Chain Reaction
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
5.3.3 Key Market Trends, Growth Factors, and Opportunities
5.3.4 Geographic Segmentation Analysis
5.4 Next Generation Sequencing
Chapter 6: Companion Diagnostics Market by Indication (2018-2032)
6.1 Companion Diagnostics Market Snapshot and Growth Engine
6.2 Market Overview
6.3 Lung Cancer
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
6.3.3 Key Market Trends, Growth Factors, and Opportunities
6.3.4 Geographic Segmentation Analysis
6.4 Breast Cancer
6.5 Colorectal Cancer
Chapter 7: Companion Diagnostics Market by End-User (2018-2032)
7.1 Companion Diagnostics Market Snapshot and Growth Engine
7.2 Market Overview
7.3 Reference Laboratories
7.3.1 Introduction and Market Overview
7.3.2 Historic and Forecasted Market Size in Value USD and Volume Units
7.3.3 Key Market Trends, Growth Factors, and Opportunities
7.3.4 Geographic Segmentation Analysis
7.4 Pharmaceutical And Biopharmaceutical Companies
Chapter 8: Company Profiles and Competitive Analysis
8.1 Competitive Landscape
8.1.1 Competitive Benchmarking
8.1.2 Companion Diagnostics Market Share by Manufacturer (2024)
8.1.3 Industry BCG Matrix
8.1.4 Heat Map Analysis
8.1.5 Mergers and Acquisitions
8.2 ABBOTT (USA)
8.2.1 Company Overview
8.2.2 Key Executives
8.2.3 Company Snapshot
8.2.4 Role of the Company in the Market
8.2.5 Sustainability and Social Responsibility
8.2.6 Operating Business Segments
8.2.7 Product Portfolio
8.2.8 Business Performance
8.2.9 Key Strategic Moves and Recent Developments
8.2.10 SWOT Analysis
8.3 AGILENT TECHNOLOGIES INC. (USA)
8.4 F. HOFFMANN-LA ROCHE LTD. (SWITZERLAND)
8.5 GUARDANT HEALTH (USA)
8.6 QIAGEN (GERMANY)
8.7 MYRIAD GENETICS INC. (USA)
8.8 ILLUMINA INC. (USA)
8.9 THERMO FISHER SCIENTIFIC INC. (USA)
8.10 BIOMERIEUX (FRANCE)
8.11 MYRIAD GENETICS INC. (USA)
8.12 OTHER ACTIVE PLAYERS
Chapter 9: Global Companion Diagnostics Market By Region
9.1 Overview
9.2. North America Companion Diagnostics Market
9.2.1 Key Market Trends, Growth Factors and Opportunities
9.2.2 Top Key Companies
9.2.3 Historic and Forecasted Market Size by Segments
9.2.4 Historic and Forecasted Market Size by Product And Service
9.2.4.1 Kits And Reagents
9.2.4.2 Software And Services
9.2.5 Historic and Forecasted Market Size by Technology
9.2.5.1 Polymerase Chain Reaction
9.2.5.2 Next Generation Sequencing
9.2.6 Historic and Forecasted Market Size by Indication
9.2.6.1 Lung Cancer
9.2.6.2 Breast Cancer
9.2.6.3 Colorectal Cancer
9.2.7 Historic and Forecasted Market Size by End-User
9.2.7.1 Reference Laboratories
9.2.7.2 Pharmaceutical And Biopharmaceutical Companies
9.2.8 Historic and Forecast Market Size by Country
9.2.8.1 US
9.2.8.2 Canada
9.2.8.3 Mexico
9.3. Eastern Europe Companion Diagnostics Market
9.3.1 Key Market Trends, Growth Factors and Opportunities
9.3.2 Top Key Companies
9.3.3 Historic and Forecasted Market Size by Segments
9.3.4 Historic and Forecasted Market Size by Product And Service
9.3.4.1 Kits And Reagents
9.3.4.2 Software And Services
9.3.5 Historic and Forecasted Market Size by Technology
9.3.5.1 Polymerase Chain Reaction
9.3.5.2 Next Generation Sequencing
9.3.6 Historic and Forecasted Market Size by Indication
9.3.6.1 Lung Cancer
9.3.6.2 Breast Cancer
9.3.6.3 Colorectal Cancer
9.3.7 Historic and Forecasted Market Size by End-User
9.3.7.1 Reference Laboratories
9.3.7.2 Pharmaceutical And Biopharmaceutical Companies
9.3.8 Historic and Forecast Market Size by Country
9.3.8.1 Russia
9.3.8.2 Bulgaria
9.3.8.3 The Czech Republic
9.3.8.4 Hungary
9.3.8.5 Poland
9.3.8.6 Romania
9.3.8.7 Rest of Eastern Europe
9.4. Western Europe Companion Diagnostics Market
9.4.1 Key Market Trends, Growth Factors and Opportunities
9.4.2 Top Key Companies
9.4.3 Historic and Forecasted Market Size by Segments
9.4.4 Historic and Forecasted Market Size by Product And Service
9.4.4.1 Kits And Reagents
9.4.4.2 Software And Services
9.4.5 Historic and Forecasted Market Size by Technology
9.4.5.1 Polymerase Chain Reaction
9.4.5.2 Next Generation Sequencing
9.4.6 Historic and Forecasted Market Size by Indication
9.4.6.1 Lung Cancer
9.4.6.2 Breast Cancer
9.4.6.3 Colorectal Cancer
9.4.7 Historic and Forecasted Market Size by End-User
9.4.7.1 Reference Laboratories
9.4.7.2 Pharmaceutical And Biopharmaceutical Companies
9.4.8 Historic and Forecast Market Size by Country
9.4.8.1 Germany
9.4.8.2 UK
9.4.8.3 France
9.4.8.4 The Netherlands
9.4.8.5 Italy
9.4.8.6 Spain
9.4.8.7 Rest of Western Europe
9.5. Asia Pacific Companion Diagnostics Market
9.5.1 Key Market Trends, Growth Factors and Opportunities
9.5.2 Top Key Companies
9.5.3 Historic and Forecasted Market Size by Segments
9.5.4 Historic and Forecasted Market Size by Product And Service
9.5.4.1 Kits And Reagents
9.5.4.2 Software And Services
9.5.5 Historic and Forecasted Market Size by Technology
9.5.5.1 Polymerase Chain Reaction
9.5.5.2 Next Generation Sequencing
9.5.6 Historic and Forecasted Market Size by Indication
9.5.6.1 Lung Cancer
9.5.6.2 Breast Cancer
9.5.6.3 Colorectal Cancer
9.5.7 Historic and Forecasted Market Size by End-User
9.5.7.1 Reference Laboratories
9.5.7.2 Pharmaceutical And Biopharmaceutical Companies
9.5.8 Historic and Forecast Market Size by Country
9.5.8.1 China
9.5.8.2 India
9.5.8.3 Japan
9.5.8.4 South Korea
9.5.8.5 Malaysia
9.5.8.6 Thailand
9.5.8.7 Vietnam
9.5.8.8 The Philippines
9.5.8.9 Australia
9.5.8.10 New Zealand
9.5.8.11 Rest of APAC
9.6. Middle East & Africa Companion Diagnostics Market
9.6.1 Key Market Trends, Growth Factors and Opportunities
9.6.2 Top Key Companies
9.6.3 Historic and Forecasted Market Size by Segments
9.6.4 Historic and Forecasted Market Size by Product And Service
9.6.4.1 Kits And Reagents
9.6.4.2 Software And Services
9.6.5 Historic and Forecasted Market Size by Technology
9.6.5.1 Polymerase Chain Reaction
9.6.5.2 Next Generation Sequencing
9.6.6 Historic and Forecasted Market Size by Indication
9.6.6.1 Lung Cancer
9.6.6.2 Breast Cancer
9.6.6.3 Colorectal Cancer
9.6.7 Historic and Forecasted Market Size by End-User
9.6.7.1 Reference Laboratories
9.6.7.2 Pharmaceutical And Biopharmaceutical Companies
9.6.8 Historic and Forecast Market Size by Country
9.6.8.1 Turkiye
9.6.8.2 Bahrain
9.6.8.3 Kuwait
9.6.8.4 Saudi Arabia
9.6.8.5 Qatar
9.6.8.6 UAE
9.6.8.7 Israel
9.6.8.8 South Africa
9.7. South America Companion Diagnostics Market
9.7.1 Key Market Trends, Growth Factors and Opportunities
9.7.2 Top Key Companies
9.7.3 Historic and Forecasted Market Size by Segments
9.7.4 Historic and Forecasted Market Size by Product And Service
9.7.4.1 Kits And Reagents
9.7.4.2 Software And Services
9.7.5 Historic and Forecasted Market Size by Technology
9.7.5.1 Polymerase Chain Reaction
9.7.5.2 Next Generation Sequencing
9.7.6 Historic and Forecasted Market Size by Indication
9.7.6.1 Lung Cancer
9.7.6.2 Breast Cancer
9.7.6.3 Colorectal Cancer
9.7.7 Historic and Forecasted Market Size by End-User
9.7.7.1 Reference Laboratories
9.7.7.2 Pharmaceutical And Biopharmaceutical Companies
9.7.8 Historic and Forecast Market Size by Country
9.7.8.1 Brazil
9.7.8.2 Argentina
9.7.8.3 Rest of SA
Chapter 10 Analyst Viewpoint and Conclusion
10.1 Recommendations and Concluding Analysis
10.2 Potential Market Strategies
Chapter 11 Research Methodology
11.1 Research Process
11.2 Primary Research
11.3 Secondary Research
Q1: What would be the forecast period in the Companion Diagnostics Market research report?
A1: The forecast period in the Companion Diagnostics Market research report is 2024-2032.
Q2: Who are the key players in the Companion Diagnostics Market?
A2: Abbott (USA), Agilent Technologies Inc. (USA), F. Hoffmann-La Roche Ltd. (Switzerland), Guardant Health (USA), QIAGEN (Germany), Myriad Genetics, Inc. (USA), Illumina Inc. (USA), Thermo Fisher Scientific Inc. (USA), BIOMERIEUX (France), Myriad Genetics Inc. (USA), Other Active Players.
Q3: What are the segments of the Companion Diagnostics Market?
A3: The Companion Diagnostics Market is segmented into Source, Application, End User and region. By Product And Service (Kits And Reagents, Software And Services), By Technology (Polymerase Chain Reaction, Next Generation Sequencing), By Indication (Lung Cancer, Breast Cancer, Colorectal Cancer), By End-User (Reference Laboratories, Pharmaceutical And Biopharmaceutical Companies). By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
Q4: What is the Companion Diagnostics Market?
A4: Companion diagnostics refers to diagnostic tests that are used in determining the presence of biomarkers in the patents for efficient decision making on the best treatment to use especially the molecular basis of many diseases today is being established. Companion diagnostics are most commonly used in oncology as they are vital for choosing patients who likely will benefit from some of the targeted treatments due to the genetic profile of the tumor. These tests are gradually becoming incorporated into patient’s management plans to make sure that the therapies that are given to patients are accorded to their specific genetic makeup, making the treatment more effective while reducing the side effects.
Q5: How big is the Companion Diagnostics Market?
A5: Companion Diagnostics Market Size Was Valued at USD 8.53 Billion in 2023, and is Projected to Reach USD 22.90 Billion by 2032, Growing at a CAGR of 11.6% From 2024-2032.
How to Buy a Report from eminsights.jp
On the product page, choose the license you want: Single-User License, Multi-User License or Enterprise License.
If you required report in your native language, then you can click on Translated Report button and fill out the form with report name and language you want, then our team will contact you as soon as possible.
Click the Buy Now button.
You will be redirected to the checkout page. Enter your company details and payment information.
Click Place Order to complete the purchase.
Confirmation: You’ll receive an order confirmation and our team will contact you shortly with your ordered report.
If you have any questions, fill out the contact form below or email us at bizdev@eminsights.net.
Thank you for choosing eminsights.jp!