Stay Ahead in Fast-Growing Economies.
Browse Reports NowAdoptive Cell Therapy Market Segment Analysis, Share, and Forecast Report (2024-2032)
The Adoptive Cell Therapy (ACT) Market is recognized to be growing at an exponential rate because of the more development in immunotherapy and the rising rate of cancer across the world. ACT is an entirely new treatment idea that involves using the patient’s own immune system in the fight against cancer.
IMR Group
Description
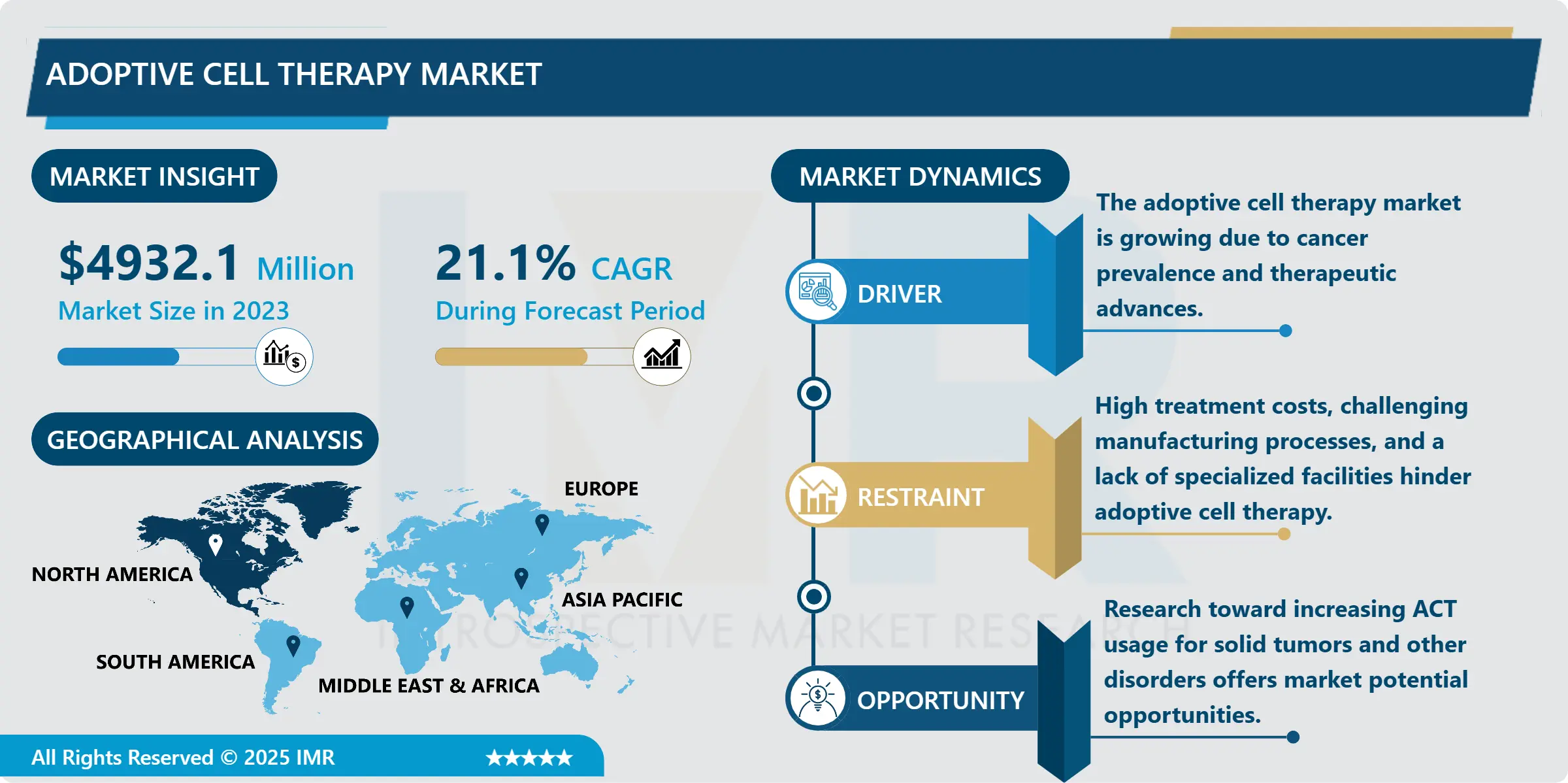
Adoptive Cell Therapy Market Synopsis:
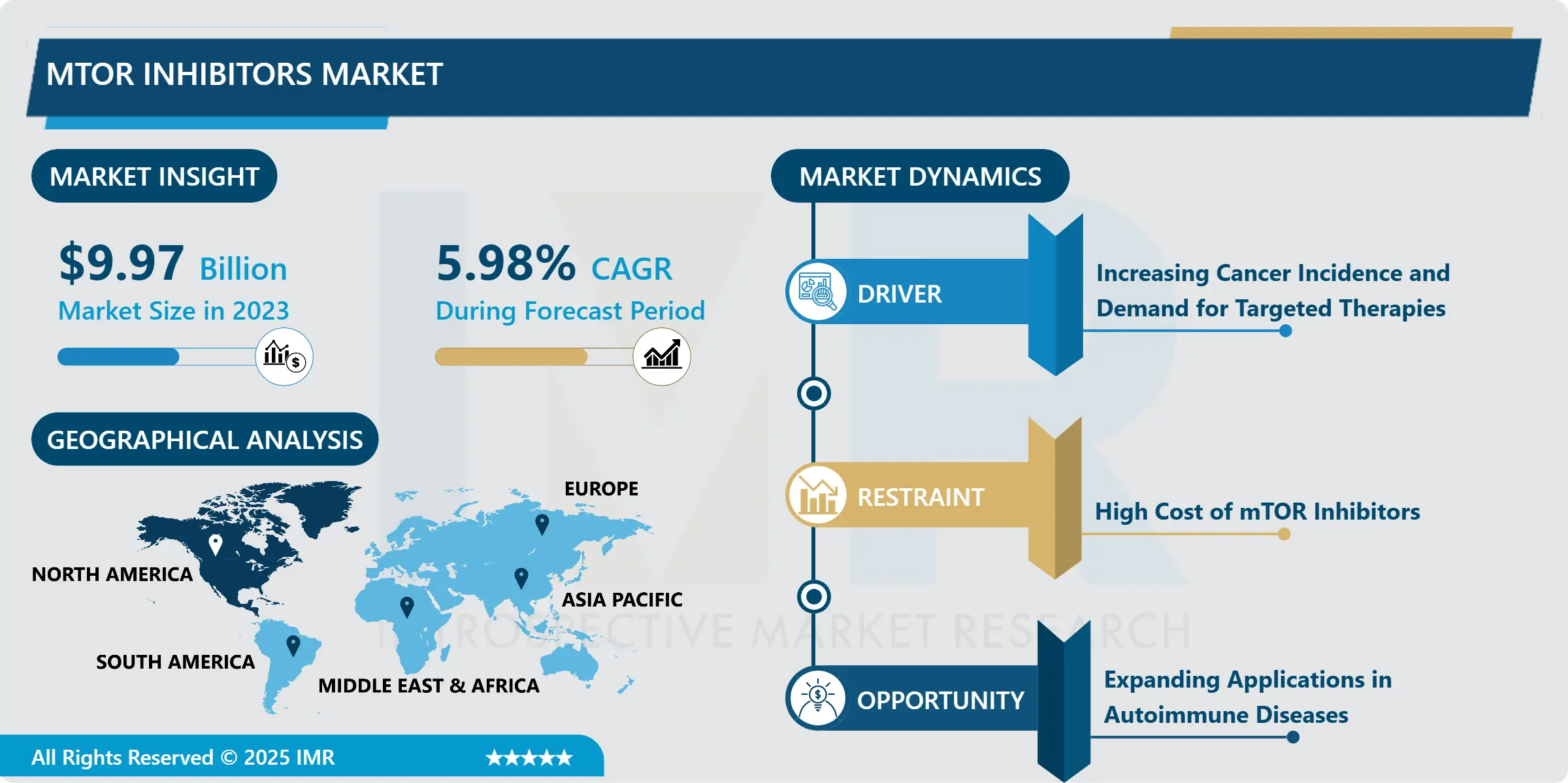
Adoptive Cell Therapy Market Size Was Valued at USD 4,932.10 Million in 2023, and is Projected to Reach USD 23,440.10 Million by 2032, Growing at a CAGR of 21.10% From 2024-2032.
The Adoptive Cell Therapy (ACT) Market is recognized to be growing at an exponential rate because of the more development in immunotherapy and the rising rate of cancer across the world. ACT is an entirely new treatment idea that involves using the patient’s own immune system in the fight against cancer. This technique involves dividing, altering, and increasing the number of distinct immune cells called T cells and then reintroducing these modified cells back into a patient to attack tumor cells. Among those, CAR T-cell therapy is the most dominant type of therapy with several FDA-approved treatments changing cancer treatment. Market growth is also sustained by a large research and development investment, and by Clinical trials evaluating the possibility of ACT for other diseases, such as viral infections and autoimmune diseases.
Demand for ACT is thus fueled by increasing patient desire for more precise treatment methods than the traditional poison like chemotherapy. Further, advancement in TILs and TCR therapies, that is new generation of ACTs is being expanded by venturing involvements between biotech companies, academic institutions and health care providers. Technological progresses in the genetic engineering or the cell manufacturing haveization are now lowering dramatically the cost of such treatments and increasing significantly the opportunities to scale up these therapies worldwide for patients.
Regionally, North America holds the largest market for ACT because of the region’s strong health care systems, high investment on the biotech research and development and first adopter of innovative therapies. Europe comes third in terms of market sales, but it exhibits a high level of support from the regulatory agencies and a strong portfolio of ACT products in the development stage. On the same note, Asia-Pacific is gradually becoming a growth-oriented market concerning the rising incidence of carcinoma, bettering health care facility structures, and raising its political science engagement in worldwide clinical trials. However, few factors like high cost of treatment, higher manufacturing costs, and issues relating to norms and standards work have to be resolved in order to maintain a continuous growth in the market.
Adoptive Cell Therapy Market Trend Analysis:
Expansion of CAR-T Therapies Beyond Hematological Cancers
One of the major emerging factors that have innovative CAR-T cell treatment expanding beyond hematological malignancies into solid tumor cancers and other diseases is a key driver within the Adoptive Cell Therapy ACT marketplace. Yescarta and Kymriah have been tremendous in managing blood cancers, but the focus now in CARTs is on the complications raised by solid tumors, including TME suppression and antigen heterogeneity. Better mechanisms such as dual-targeting CAR-T cells and the armored CAR-T cells are other emerge technologies together with advanced gene editing methods that are producing better outputs in different types of cancer. It is believed that these progresses will expand the category of diseases that can be treated using CAR-T therapies and, thus, will contribute to the further development of the market.
Advancements in Tumor-Infiltrating Lymphocyte (TIL) Therapy
Another trend of increasing concern is the need for Tumor-Infiltrating Lymphocyte (TIL) therapy, which involves a patient’s T cells sourced from the tumors. TIL therapy has proven to be effective for solid tumor, especially melanoma since it can take on several tumor antigens at a time. Organizations are now spending their resources to enhance TIL manufacturing procedures for lowering the treatment durations. Furthermore, molecular characterization of post-TIL outcomes and combinations of TIL therapy with immune checkpoint inhibitors are under investigation to improve the treatment outcomes. Therefore, TIL therapy is already paving way to become an affordable option when compared to CAR-T therapies which in a way is going to make the ACT market and more importantly treatment options even richer for patients.
Adoptive Cell Therapy Market Segment Analysis:
Adoptive Cell Therapy Market is Segmented on the basis of Type, Application, End User, and Region.
By Type, CAR-T segment is expected to dominate the market during the forecast period
The adoptive cell therapy market can be split by type: CAR-T cells therapy, TCR-T cells therapy, NK cells therapy, and TIL cells therapy, each type has its therapeutic benefits. These are dominated by CAR-T therapies due to the high response they have shown towards treating haematological cancers and the constant effort being made to improve the therapies for solid tumors as well. Like any other immunotherapies, TCR-T therapies have been developed to harness the strength of T cells receptors to identify intracellular tumor antigens. while currently being popular for their targeted nature, more development is expected to make them a solution for various types of cancer. NK cells are establishing themselves as the optimistic off-the-shelf carriers because of their inborn immune response mechanisms; not limited to the cancer therapies but embracing the infectious ailments too. On the other hand, TIL therapies are tasked with utilizing lymphocytes that are already present in the body, present a great potential particularly in melanoma, which is a solid tumor. Such diverse segmentation points to an increasing trend in the personalized and innovative market for immunotherapy.
By End User, Hospitals segment expected to held the largest share
The Adoptive Cell Therapy (ACT) Market organized by end users shows that hospitals and cancer treatment centers remain at the forefront of delivering these innovative cellular therapies to patients. Due to the important role they advance in the health care systems, hospitals help drive ACT treatments and especially for intricate cases involving cell therapies like CAR-T cell therapy which require the support of physicians from different disciplines and complex facilities. Cancer treatment centers, conversely, pride themselves on providing patients with comprehensive and sometimes experimental ACT, as many cancer treatment centers perform clinical trials and offer state-of-art TIL and TCR therapies. These centers are mostly concerned with the treatment approaches that are tailored to meet an individual patient’s needs hence patients who want a new approach seek them. Such collaboration between these bioscience firms and these institutions is further making ACCESS AND EXPERTISE to ACT available and advanced thus contributing to the market growth of ACT.
Adoptive Cell Therapy Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
North America is anticipated to command the Adoptive Cell Therapy (ACT) Market throughout the forecast period due to existence of well-developed healthcare system, favorable investment in biotechnology, and significant use of novel treatment such as CAR-T cell and TIL therapies. ACT has significant governmental support and favorable legislation for the development of the market in the region, coupled with the engagement of industrial stakeholders in the commercialization of products for ACT. Further, the incidence of cancer and rising demand for a tailored medical approach to diseases are the factors that drive the growth of the overall market. Strategic partnerships between different academic institutions and biotechnology companies are increasing innovation and the abundance of clinical trials make North America at the forefront in the ongoing development of solution-focused ACT worldwide.
Active Key Players in the Adoptive Cell Therapy Market
Arcellx (USA)
Biodesix, Inc. (USA)
bluebird bio, Inc. (USA)
Castle Creek Biosciences, Inc. (USA)
Cellectis (France)
Gilead Sciences, Inc. (USA)
ImmunityBio, Inc. (USA)
Laurus Labs (India)
Lineage Cell Therapeutics, Inc. (USA)
Novartis AG (Switzerland)
Sana Biotechnology, Inc. (USA)
Sorrento Therapeutics (USA)
Transgene SA (France)
Other Active Players.
Chapter 1: Introduction
1.1 Scope and Coverage
Chapter 2:Executive Summary
Chapter 3: Market Landscape
3.1 Market Dynamics
3.1.1 Drivers
3.1.2 Restraints
3.1.3 Opportunities
3.1.4 Challenges
3.2 Market Trend Analysis
3.3 PESTLE Analysis
3.4 Porter’s Five Forces Analysis
3.5 Industry Value Chain Analysis
3.6 Ecosystem
3.7 Regulatory Landscape
3.8 Price Trend Analysis
3.9 Patent Analysis
3.10 Technology Evolution
3.11 Investment Pockets
3.12 Import-Export Analysis
Chapter 4: Adoptive Cell Therapy Market by Type
4.1 Adoptive Cell Therapy Market Snapshot and Growth Engine
4.2 Adoptive Cell Therapy Market Overview
4.3 CAR-T
4.3.1 Introduction and Market Overview
4.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.3.3 Key Market Trends, Growth Factors and Opportunities
4.3.4 CAR-T: Geographic Segmentation Analysis
4.4 TCR-T
4.4.1 Introduction and Market Overview
4.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.4.3 Key Market Trends, Growth Factors and Opportunities
4.4.4 TCR-T: Geographic Segmentation Analysis
4.5 NK
4.5.1 Introduction and Market Overview
4.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.5.3 Key Market Trends, Growth Factors and Opportunities
4.5.4 NK: Geographic Segmentation Analysis
4.6 and TIL)
4.6.1 Introduction and Market Overview
4.6.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
4.6.3 Key Market Trends, Growth Factors and Opportunities
4.6.4 and TIL): Geographic Segmentation Analysis
Chapter 5: Adoptive Cell Therapy Market by Application
5.1 Adoptive Cell Therapy Market Snapshot and Growth Engine
5.2 Adoptive Cell Therapy Market Overview
5.3 Lymphoma
5.3.1 Introduction and Market Overview
5.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.3.3 Key Market Trends, Growth Factors and Opportunities
5.3.4 Lymphoma: Geographic Segmentation Analysis
5.4 Leukemia
5.4.1 Introduction and Market Overview
5.4.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.4.3 Key Market Trends, Growth Factors and Opportunities
5.4.4 Leukemia: Geographic Segmentation Analysis
5.5 and Others
5.5.1 Introduction and Market Overview
5.5.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
5.5.3 Key Market Trends, Growth Factors and Opportunities
5.5.4 and Others: Geographic Segmentation Analysis
Chapter 6: Adoptive Cell Therapy Market by End User
6.1 Adoptive Cell Therapy Market Snapshot and Growth Engine
6.2 Adoptive Cell Therapy Market Overview
6.3 Hospitals and Cancer Treatment Centers
6.3.1 Introduction and Market Overview
6.3.2 Historic and Forecasted Market Size in Value USD and Volume Units (2017-2032F)
6.3.3 Key Market Trends, Growth Factors and Opportunities
6.3.4 Hospitals and Cancer Treatment Centers: Geographic Segmentation Analysis
Chapter 7: Company Profiles and Competitive Analysis
7.1 Competitive Landscape
7.1.1 Competitive Benchmarking
7.1.2 Adoptive Cell Therapy Market Share by Manufacturer (2023)
7.1.3 Industry BCG Matrix
7.1.4 Heat Map Analysis
7.1.5 Mergers and Acquisitions
7.2 NOVARTIS AG (SWITZERLAND)
7.2.1 Company Overview
7.2.2 Key Executives
7.2.3 Company Snapshot
7.2.4 Role of the Company in the Market
7.2.5 Sustainability and Social Responsibility
7.2.6 Operating Business Segments
7.2.7 Product Portfolio
7.2.8 Business Performance
7.2.9 Key Strategic Moves and Recent Developments
7.2.10 SWOT Analysis
7.3 GILEAD SCIENCES INC. (USA)
7.4 CASTLE CREEK BIOSCIENCES INC. (USA)
7.5 LINEAGE CELL THERAPEUTICS INC. (USA)
7.6 TRANSGENE SA (FRANCE)
7.7 CELLECTIS (FRANCE)
7.8 IMMUNITYBIO INC. (USA)
7.9 SORRENTO THERAPEUTICS (USA)
7.10 BLUEBIRD BIO INC. (USA)
7.11 ARCELLX (USA)
7.12 SANA BIOTECHNOLOGY INC. (USA)
7.13 BIODESIX INC. (USA)
7.14 LAURUS LABS (INDIA)
7.15 OTHER ACTIVE PLAYERS
Chapter 8: Global Adoptive Cell Therapy Market By Region
8.1 Overview
8.2. North America Adoptive Cell Therapy Market
8.2.1 Key Market Trends, Growth Factors and Opportunities
8.2.2 Top Key Companies
8.2.3 Historic and Forecasted Market Size by Segments
8.2.4 Historic and Forecasted Market Size By Type
8.2.4.1 CAR-T
8.2.4.2 TCR-T
8.2.4.3 NK
8.2.4.4 and TIL)
8.2.5 Historic and Forecasted Market Size By Application
8.2.5.1 Lymphoma
8.2.5.2 Leukemia
8.2.5.3 and Others
8.2.6 Historic and Forecasted Market Size By End User
8.2.6.1 Hospitals and Cancer Treatment Centers
8.2.7 Historic and Forecast Market Size by Country
8.2.7.1 US
8.2.7.2 Canada
8.2.7.3 Mexico
8.3. Eastern Europe Adoptive Cell Therapy Market
8.3.1 Key Market Trends, Growth Factors and Opportunities
8.3.2 Top Key Companies
8.3.3 Historic and Forecasted Market Size by Segments
8.3.4 Historic and Forecasted Market Size By Type
8.3.4.1 CAR-T
8.3.4.2 TCR-T
8.3.4.3 NK
8.3.4.4 and TIL)
8.3.5 Historic and Forecasted Market Size By Application
8.3.5.1 Lymphoma
8.3.5.2 Leukemia
8.3.5.3 and Others
8.3.6 Historic and Forecasted Market Size By End User
8.3.6.1 Hospitals and Cancer Treatment Centers
8.3.7 Historic and Forecast Market Size by Country
8.3.7.1 Russia
8.3.7.2 Bulgaria
8.3.7.3 The Czech Republic
8.3.7.4 Hungary
8.3.7.5 Poland
8.3.7.6 Romania
8.3.7.7 Rest of Eastern Europe
8.4. Western Europe Adoptive Cell Therapy Market
8.4.1 Key Market Trends, Growth Factors and Opportunities
8.4.2 Top Key Companies
8.4.3 Historic and Forecasted Market Size by Segments
8.4.4 Historic and Forecasted Market Size By Type
8.4.4.1 CAR-T
8.4.4.2 TCR-T
8.4.4.3 NK
8.4.4.4 and TIL)
8.4.5 Historic and Forecasted Market Size By Application
8.4.5.1 Lymphoma
8.4.5.2 Leukemia
8.4.5.3 and Others
8.4.6 Historic and Forecasted Market Size By End User
8.4.6.1 Hospitals and Cancer Treatment Centers
8.4.7 Historic and Forecast Market Size by Country
8.4.7.1 Germany
8.4.7.2 UK
8.4.7.3 France
8.4.7.4 The Netherlands
8.4.7.5 Italy
8.4.7.6 Spain
8.4.7.7 Rest of Western Europe
8.5. Asia Pacific Adoptive Cell Therapy Market
8.5.1 Key Market Trends, Growth Factors and Opportunities
8.5.2 Top Key Companies
8.5.3 Historic and Forecasted Market Size by Segments
8.5.4 Historic and Forecasted Market Size By Type
8.5.4.1 CAR-T
8.5.4.2 TCR-T
8.5.4.3 NK
8.5.4.4 and TIL)
8.5.5 Historic and Forecasted Market Size By Application
8.5.5.1 Lymphoma
8.5.5.2 Leukemia
8.5.5.3 and Others
8.5.6 Historic and Forecasted Market Size By End User
8.5.6.1 Hospitals and Cancer Treatment Centers
8.5.7 Historic and Forecast Market Size by Country
8.5.7.1 China
8.5.7.2 India
8.5.7.3 Japan
8.5.7.4 South Korea
8.5.7.5 Malaysia
8.5.7.6 Thailand
8.5.7.7 Vietnam
8.5.7.8 The Philippines
8.5.7.9 Australia
8.5.7.10 New Zealand
8.5.7.11 Rest of APAC
8.6. Middle East & Africa Adoptive Cell Therapy Market
8.6.1 Key Market Trends, Growth Factors and Opportunities
8.6.2 Top Key Companies
8.6.3 Historic and Forecasted Market Size by Segments
8.6.4 Historic and Forecasted Market Size By Type
8.6.4.1 CAR-T
8.6.4.2 TCR-T
8.6.4.3 NK
8.6.4.4 and TIL)
8.6.5 Historic and Forecasted Market Size By Application
8.6.5.1 Lymphoma
8.6.5.2 Leukemia
8.6.5.3 and Others
8.6.6 Historic and Forecasted Market Size By End User
8.6.6.1 Hospitals and Cancer Treatment Centers
8.6.7 Historic and Forecast Market Size by Country
8.6.7.1 Turkiye
8.6.7.2 Bahrain
8.6.7.3 Kuwait
8.6.7.4 Saudi Arabia
8.6.7.5 Qatar
8.6.7.6 UAE
8.6.7.7 Israel
8.6.7.8 South Africa
8.7. South America Adoptive Cell Therapy Market
8.7.1 Key Market Trends, Growth Factors and Opportunities
8.7.2 Top Key Companies
8.7.3 Historic and Forecasted Market Size by Segments
8.7.4 Historic and Forecasted Market Size By Type
8.7.4.1 CAR-T
8.7.4.2 TCR-T
8.7.4.3 NK
8.7.4.4 and TIL)
8.7.5 Historic and Forecasted Market Size By Application
8.7.5.1 Lymphoma
8.7.5.2 Leukemia
8.7.5.3 and Others
8.7.6 Historic and Forecasted Market Size By End User
8.7.6.1 Hospitals and Cancer Treatment Centers
8.7.7 Historic and Forecast Market Size by Country
8.7.7.1 Brazil
8.7.7.2 Argentina
8.7.7.3 Rest of SA
Chapter 9 Analyst Viewpoint and Conclusion
9.1 Recommendations and Concluding Analysis
9.2 Potential Market Strategies
Chapter 10 Research Methodology
10.1 Research Process
10.2 Primary Research
10.3 Secondary Research
Q1: What would be the forecast period in the Adoptive Cell Therapy Market research report?
A1: The forecast period in the Adoptive Cell Therapy Market research report is 2024-2032.
Q2: Who are the key players in the Adoptive Cell Therapy Market?
A2: Novartis AG (Switzerland), Gilead Sciences, Inc. (USA), Castle Creek Biosciences, Inc. (USA), Lineage Cell Therapeutics, Inc. (USA), Transgene SA (France), Cellectis (France), ImmunityBio, Inc. (USA), Sorrento Therapeutics (USA), bluebird bio, Inc. (USA), Arcellx (USA), Sana Biotechnology, Inc. (USA), Biodesix, Inc. (USA), Laurus Labs (India), Other Active Players.
Q3: What are the segments of the Adoptive Cell Therapy Market?
A3: The Aircraft Cabin Interior Market is segmented into by Type, By Application, End User and region. by Type (CAR-T, TCR-T, NK, and TIL), By Application (Lymphoma, Leukemia, and Others), by End User (Hospitals and Cancer Treatment Centers). By region, it is analyzed across North America (U.S., Canada, Mexico), Eastern Europe (Russia, Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe), Western Europe (Germany, UK, France, The Netherlands, Italy, Russia, Spain, Rest of Western Europe), Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC), Middle East & Africa (Turkiye, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa), South America (Brazil, Argentina, Rest of SA).
Q4: What is the Adoptive Cell Therapy Market?
A4: Adoptive Cell Therapy (ACT) is an advanced immunotherapy that involves the infusion of modified or expanded immune cells to treat various diseases, particularly cancer. The process typically involves extracting immune cells, such as T cells, from a patient’s body, genetically modifying or enhancing them to better recognize and attack cancer cells, and then reintroducing these cells into the patient's bloodstream. ACT includes therapies like CAR-T (Chimeric Antigen Receptor T-cell therapy), where T cells are engineered to target specific cancer markers, and Tumor-Infiltrating Lymphocyte (TIL) therapy, which utilizes T cells from the tumor itself. This approach aims to harness and amplify the body’s natural immune response to fight cancer more effectively, offering a targeted, personalized alternative to traditional treatments like chemotherapy and radiation.
Q5: How big is the Adoptive Cell Therapy Market?
A5: Adoptive Cell Therapy Market Size Was Valued at USD 4,932.10 Million in 2023, and is Projected to Reach USD 23,440.10 Million by 2032, Growing at a CAGR of 21.10% From 2024-2032.
How to Buy a Report from eminsights.jp
On the product page, choose the license you want: Single-User License, Multi-User License or Enterprise License.
If you required report in your native language, then you can click on Translated Report button and fill out the form with report name and language you want, then our team will contact you as soon as possible.
Click the Buy Now button.
You will be redirected to the checkout page. Enter your company details and payment information.
Click Place Order to complete the purchase.
Confirmation: You’ll receive an order confirmation and our team will contact you shortly with your ordered report.
If you have any questions, fill out the contact form below or email us at bizdev@eminsights.net.
Thank you for choosing eminsights.jp!